Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (11): 3668-3683.DOI: 10.6023/cjoc202205049 Previous Articles Next Articles
ARTICLES
张涛a, 卫海沅a, 马雯a, 李张媛a, 胡盼盼a, 周楠茜b, 贺建超a, 李婷a, 苏明明a, 白素平a,*( )
)
收稿日期:2022-05-28
修回日期:2022-08-01
发布日期:2022-08-17
通讯作者:
白素平
基金资助:
Tao Zhanga, Haiyuan Weia, Wen Maa, Zhangyuan Lia, Panpan Hua, Nanqian Zhoub, Jianchao Hea, Ting Lia, Mingming Sua, Suping Baia( )
)
Received:2022-05-28
Revised:2022-08-01
Published:2022-08-17
Contact:
Suping Bai
Supported by:Share
Tao Zhang, Haiyuan Wei, Wen Ma, Zhangyuan Li, Panpan Hu, Nanqian Zhou, Jianchao He, Ting Li, Mingming Su, Suping Bai. Synthesis and Antiproliferative Activity Evaluation of Novel Glaucocalyxin A-1,2,3-Triazole Derivatives[J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3668-3683.

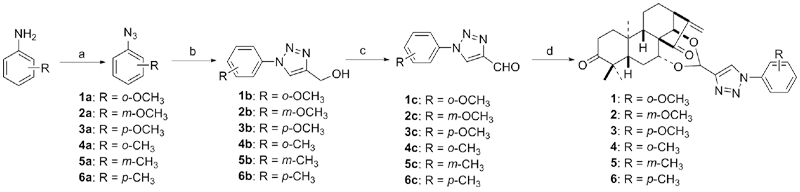

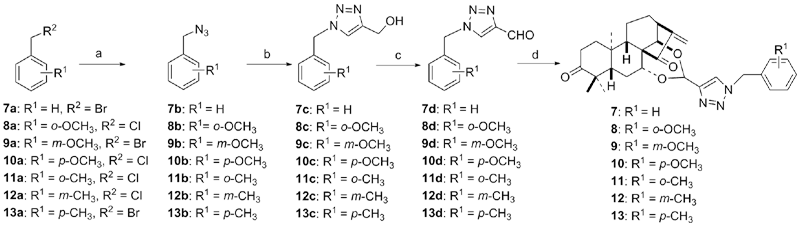
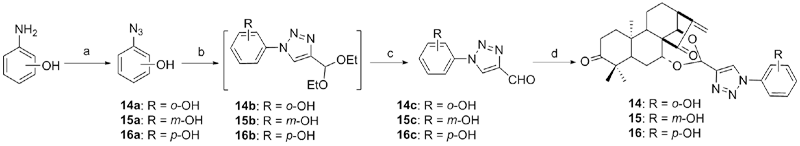
| Compd. | HepG2 | NCI-H460 | JEG-3 | K562 | HL-60 | Hela |
|---|---|---|---|---|---|---|
| 1 | 5.78±0.10 | 8.30±0.38 | 5.15±0.26 | 8.28±0.15 | 4.52±0.17 | 5.85±0.61 |
| 2 | 21.57±0.39 | 13.46±0.61 | 3.22±0.25 | 14.52±0.19 | 2.29±0.10 | 15.12±0.59 |
| 3 | 30.38±0.18 | 80.34±2.96 | 5.32±0.30 | 60.62±0.62 | 24.05±2.46 | 16.39±0.23 |
| 4 | 2.89±0.01 | 21.71±0.17 | 4.15±0.04 | 9.03±0.06 | 1.98±0.13 | 2.77±0.06 |
| 5 | 25.27±2.06 | 31.87±0.16 | 14.94±1.90 | 28.71±2.16 | 14.75±0.35 | 14.19±0.97 |
| 6 | >100 | >100 | >100 | >100 | 29.23±0.29 | 72.13±2.40 |
| 7 | 13.69±0.13 | 21.34±1.03 | 9.51±0.15 | 32.00±0.38 | 14.28±1.51 | 12.32±0.21 |
| 8 | 1.85±0.28 | 4.66±0.09 | 2.53±0.02 | 5.04±0.26 | 1.97±0.06 | 1.59±0.09 |
| 9 | 12.23±1.06 | 29.33±0.59 | 5.50±0.04 | 19.69±0.24 | 11.55±1.32 | 7.88±0.13 |
| 10 | 3.03±0.14 | 4.70±0.05 | 2.86±0.07 | 5.40±0.40 | 2.40±0.11 | 3.28±0.06 |
| 11 | 5.83±0.14 | 9.68±0.19 | 2.93±0.17 | 13.77±0.27 | 3.22±0.19 | 5.56±0.57 |
| 12 | 8.05±0.31 | 18.44±0.55 | 1.66±0.03 | 18.29±0.94 | 12.37±0.50 | 16.27±0.61 |
| 13 | 5.45±0.24 | 13.09±1.02 | 12.49±0.23 | 18.30±0.55 | 5.96±0.42 | 7.45±0.12 |
| 14 | 16.42±0.45 | 13.27±0.28 | 5.07±0.05 | 67.11±0.91 | 1.18±0.02 | 2.15±0.18 |
| 15 | 2.53±0.14 | 24.52±0.26 | 6.21±0.10 | 17.05±0.76 | 0.36±0.02 | 6.78±0.10 |
| 16 | 1.43±0.04 | 5.56±0.06 | 1.18±0.05 | 5.08±0.22 | 0.25±0.01 | 2.85±0.03 |
| 17 | 18.19±1.11 | 16.61±1.00 | 18.39±0.53 | >100 | 4.73±0.36 | 10.60±0.45 |
| 18 | 4.49±0.08 | 2.85±0.07 | 1.47±0.25 | 3.77±0.10 | 1.16±0.12 | 3.00±0.14 |
| 19 | 12.16±0.64 | 7.56±0.11 | 20.18±0.32 | 14.14±0.88 | 2.53±0.12 | 5.06±0.22 |
| 20 | 11.98±0.14 | 10.87±0.84 | 9.92±0.82 | 16.35±0.54 | 4.75±0.03 | 4.64±0.10 |
| 21 | 21.88±1.01 | 51.64±0.37 | 19.59±0.38 | 81.4±4.61 | >100 | 29.61±0.84 |
| 22 | 6.81±0.53 | 4.92±0.17 | 6.40±0.18 | 16.03±0.45 | 3.47±0.05 | 4.27±0.11 |
| GLA | 3.94±0.07 | 8.99±0.18 | 4.25±0.08 | 7.20±0.17 | 6.46±0.05 | 8.19±1.96 |
| Adriamycin | 0.50±0.01 | 0.29±0.02 | 0.52±0.02 | 5.15±0.11 | 1.73±0.02 | 0.69±0.01 |
| Compd. | HepG2 | NCI-H460 | JEG-3 | K562 | HL-60 | Hela |
|---|---|---|---|---|---|---|
| 1 | 5.78±0.10 | 8.30±0.38 | 5.15±0.26 | 8.28±0.15 | 4.52±0.17 | 5.85±0.61 |
| 2 | 21.57±0.39 | 13.46±0.61 | 3.22±0.25 | 14.52±0.19 | 2.29±0.10 | 15.12±0.59 |
| 3 | 30.38±0.18 | 80.34±2.96 | 5.32±0.30 | 60.62±0.62 | 24.05±2.46 | 16.39±0.23 |
| 4 | 2.89±0.01 | 21.71±0.17 | 4.15±0.04 | 9.03±0.06 | 1.98±0.13 | 2.77±0.06 |
| 5 | 25.27±2.06 | 31.87±0.16 | 14.94±1.90 | 28.71±2.16 | 14.75±0.35 | 14.19±0.97 |
| 6 | >100 | >100 | >100 | >100 | 29.23±0.29 | 72.13±2.40 |
| 7 | 13.69±0.13 | 21.34±1.03 | 9.51±0.15 | 32.00±0.38 | 14.28±1.51 | 12.32±0.21 |
| 8 | 1.85±0.28 | 4.66±0.09 | 2.53±0.02 | 5.04±0.26 | 1.97±0.06 | 1.59±0.09 |
| 9 | 12.23±1.06 | 29.33±0.59 | 5.50±0.04 | 19.69±0.24 | 11.55±1.32 | 7.88±0.13 |
| 10 | 3.03±0.14 | 4.70±0.05 | 2.86±0.07 | 5.40±0.40 | 2.40±0.11 | 3.28±0.06 |
| 11 | 5.83±0.14 | 9.68±0.19 | 2.93±0.17 | 13.77±0.27 | 3.22±0.19 | 5.56±0.57 |
| 12 | 8.05±0.31 | 18.44±0.55 | 1.66±0.03 | 18.29±0.94 | 12.37±0.50 | 16.27±0.61 |
| 13 | 5.45±0.24 | 13.09±1.02 | 12.49±0.23 | 18.30±0.55 | 5.96±0.42 | 7.45±0.12 |
| 14 | 16.42±0.45 | 13.27±0.28 | 5.07±0.05 | 67.11±0.91 | 1.18±0.02 | 2.15±0.18 |
| 15 | 2.53±0.14 | 24.52±0.26 | 6.21±0.10 | 17.05±0.76 | 0.36±0.02 | 6.78±0.10 |
| 16 | 1.43±0.04 | 5.56±0.06 | 1.18±0.05 | 5.08±0.22 | 0.25±0.01 | 2.85±0.03 |
| 17 | 18.19±1.11 | 16.61±1.00 | 18.39±0.53 | >100 | 4.73±0.36 | 10.60±0.45 |
| 18 | 4.49±0.08 | 2.85±0.07 | 1.47±0.25 | 3.77±0.10 | 1.16±0.12 | 3.00±0.14 |
| 19 | 12.16±0.64 | 7.56±0.11 | 20.18±0.32 | 14.14±0.88 | 2.53±0.12 | 5.06±0.22 |
| 20 | 11.98±0.14 | 10.87±0.84 | 9.92±0.82 | 16.35±0.54 | 4.75±0.03 | 4.64±0.10 |
| 21 | 21.88±1.01 | 51.64±0.37 | 19.59±0.38 | 81.4±4.61 | >100 | 29.61±0.84 |
| 22 | 6.81±0.53 | 4.92±0.17 | 6.40±0.18 | 16.03±0.45 | 3.47±0.05 | 4.27±0.11 |
| GLA | 3.94±0.07 | 8.99±0.18 | 4.25±0.08 | 7.20±0.17 | 6.46±0.05 | 8.19±1.96 |
| Adriamycin | 0.50±0.01 | 0.29±0.02 | 0.52±0.02 | 5.15±0.11 | 1.73±0.02 | 0.69±0.01 |


| [1] |
Newman, D. J.; Cragg, G. M. J. Nat. Prod. 2020, 83, 770.
doi: 10.1021/acs.jnatprod.9b01285 pmid: 32162523 |
| [2] |
Atanasov, A. G.; Zotchev, S. B.; Dirsch, V. M.; Orhan, I. E.; Banach, M.; Rollinger, J. M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E. A.; Majeed, M.; Bishayee, A.; Bochkov, V.; Bonn, G. K.; Braidy, N.; Bucar, F.; Cifuentes, A.; D’Onofrio, G.; Bodkin, M.; Diederich, M.; Dinkova-Kostova, A. T.; Efferth, T.; El Bairi, K.; Arkells, N.; Fan, T.-P.; Fiebich, B. L.; Freissmuth, M.; Georgiev, M. I.; Gibbons, S.; Godfrey, K. M.; Gruber, C. W.; Heer, J.; Huber, L. A.; Ibanez, E.; Kijjoa, A.; Kiss, A. K.; Lu, A.; Macias, F. A.; Miller, M. J. S.; Mocan, A.; Müller, R.; Nicoletti, F.; Perry, G.; Pittalà, V.; Rastrelli, L.; Ristow, M.; Russo, G. L.; Silva, A. S.; Schuster, D.; Sheridan, H.; Skalicka-Woźniak, K.; Skaltsounis, L.; Sobarzo- Sánchez, E.; Bredt, D. S.; Stuppner, H.; Sureda, A.; Tzvetkov, N. T.; Vacca, R. A.; Aggarwal, B. B.; Battino, M.; Giampieri, F.; Wink, M.; Wolfender, J.-L.; Xiao, J.; Yeung, A. W. K.; Lizard, G.; Popp, M. A.; Heinrich, M.; Berindan-Neagoe, I.; Stadler, M.; Daglia, M.; Verpoorte, R.; Supuran, C. T. Nat. Rev. Drug Discovery 2021, 20, 200.
doi: 10.1038/s41573-020-00114-z |
| [3] |
Xiang, Z.; Wu, X.; Liu, X.; Jin, Y. Nat. Prod. Res. 2014, 28, 2221.
doi: 10.1080/14786419.2014.934235 |
| [4] |
Li, D.; Han, T.; Liao, J.; Hu, X.; Xu, S.; Tian, K.; Gu, X.; Cheng, K.; Li, Z.; Hua, H.; Xu, J. Int. J. Mol. Sci. 2016, 17, 1395.
doi: 10.3390/ijms17091395 |
| [5] |
Wang, M.; Li, H.; Xu, F.; Gao, X.; Li, J.; Xu, S.; Zhang, D.; Wu, X.; Xu, J.; Hua, H.; Li, D. Eur. J. Med. Chem. 2018, 156, 885.
doi: 10.1016/j.ejmech.2018.07.052 |
| [6] |
Nagashima, F.; Kondoh, M.; Fujii, M.; Takaoka, S.; Watanabe, Y.; Asakawa, Y. Tetrahedron 2005, 61, 4531.
doi: 10.1016/j.tet.2005.03.010 |
| [7] |
Huang, S.-X.; Zhao, Q.-S.; Xu, G.; Xiao, W.-L.; Li, R.-T.; Hou, A.-J.; Peng, S.-L.; Ding, L.-S.; Sun, H.-D. J. Nat. Prod. 2005, 68, 1758.
doi: 10.1021/np050342o |
| [8] |
Li, X.; Xiao, W.; Pu, J.; Ban, L.; Shen, Y.; Weng, Z.; Li, S.; Sun, H. Phytochemistry 2006, 67, 1336.
doi: 10.1016/j.phytochem.2006.05.002 |
| [9] |
Chen, S.; Liu, J.; Zhang, H. J. Huazhong Univ. Sci. Technol., Med. Sci. 2009, 29, 659.
doi: 10.1016/0277-9536(89)90186-X |
| [10] |
Liang, H.-J.; Zhang, Y.-X.; Hai, G.-F.; Bai, S.-P.; Yuan, Y.-L.; Ye, D.-D.; Zhou, N.-Q. Planta Med. 2012, 78, 589.
doi: 10.1055/s-0031-1298265 |
| [11] |
Zhang, J.-X.; Han, Q.-B.; Zhao, A.-H.; Sun, H.-D. Fitoterapia 2003, 74, 435.
pmid: 12837357 |
| [12] |
Zhao, Y.; Niu, X.-M.; Qian, L.-P.; Liu, Z.-Y.; Zhao, Q.-S. Eur. J. Med. Chem. 2007, 42, 494.
pmid: 17189663 |
| [13] |
Liu, G.; Ding, L.; Yang, Y.; Yang, H.; Yang, Q.; Wang, H. Res. Chem. Intermed. 2006, 32, 787.
doi: 10.1163/156856706778606543 |
| [14] |
Zhang, T.; Li, N. X.; Zhou, N. Q.; Ma, W.; Wei, H. Y.; Zhang, B. X.; Chen, L. H.; Hai, G. F.; Duan, Y. C.; Bai, S. P. Chin. J. Org. Chem. 2021, 41, 2393. (in Chinese)
doi: 10.6023/cjoc202101058 |
|
( 张涛, 李念先, 周楠茜, 马雯, 卫海沅, 张冰欣, 陈亮辉, 海广范, 段迎超, 白素平, 有机化学 2021, 41, 2393.)
doi: 10.6023/cjoc202101058 |
|
| [15] |
Chen, J.; Zhang, W.; Pan, C.; Fan, J.; Zhong, X.; Tang, S. Life Sci. 2021, 271, 119185.
doi: 10.1016/j.lfs.2021.119185 |
| [16] |
Wang, X.; He, M.-J.; Chen, X.-J.; Bai, Y.-T.; Zhou, G. J. Ethnopharmacol. 2022, 290, 115100.
doi: 10.1016/j.jep.2022.115100 |
| [17] |
Ren, L.; Wang, J.; Chen, G. Drug Delivery 2019, 26, 309.
doi: 10.1080/10717544.2019.1568623 |
| [18] |
Boechat, N.; Ferreira, V. F.; Ferreira, S. B.; Ferreira, M. d. L. G.; da Silva, F. d. C.; Bastos, M. M.; Costa, M. d. S.; Lourenço, M. C. S.; Pinto, A. C.; Krettli, A. U.; Aguiar, A. C.; Teixeira, B. M.; da Silva, N. V.; Martins, P. R. C.; Bezerra, F. A. F. M.; Camilo, A. L. S.; da Silva, G. P.; Costa, C. C. P. J. Med. Chem. 2011, 54, 5988.
doi: 10.1021/jm2003624 pmid: 21776985 |
| [19] |
Bozorov, K.; Zhao, J.; Aisa, H. A. Bioorg. Med. Chem. 2019, 27, 3511.
|
| [20] |
Xu, Z.; Zhao, S.-J.; Liu, Y. Eur. J. Med. Chem. 2019, 183, 111700.
doi: 10.1016/j.ejmech.2019.111700 |
| [21] |
Ke, Y.; Wang, W.; Zhao, L.-F.; Liang, J.-J.; Liu, Y.; Zhang, X.; Feng, K.; Liu, H.-M. Bioorg. Med. Chem. 2018, 26, 4761.
doi: 10.1016/j.bmc.2017.11.005 |
| [22] |
Ke, Y.; Liang, J.-J.; Hou, R.-J.; Li, M.-M.; Zhao, L.-F.; Wang, W.; Liu, Y.; Xie, H.; Yang, R.-H.; Hu, T.-X.; Wang, J.-Y.; Liu, H.-M. Eur. J. Med. Chem. 2018, 157, 1249.
doi: 10.1016/j.ejmech.2018.08.056 |
| [23] |
Andreeva, O. V.; Garifullin, B. F.; Sharipova, R. R.; Strobykina, I. Y.; Sapunova, A. S.; Voloshina, A. D.; Belenok, M. G.; Dobrynin, A. B.; Khabibulina, L. R.; Kataev, V. E. J. Nat. Prod. 2020, 83, 2367.
doi: 10.1021/acs.jnatprod.0c00134 pmid: 32786882 |
| [24] |
Liang, L.; Astruc, D. Coord. Chem. Rev. 2011, 255, 2933.
doi: 10.1016/j.ccr.2011.06.028 |
| [25] |
Sun, S.; Wu, P. J. Phys. Chem. A 2010, 114, 8331.
doi: 10.1021/jp105034m |
| [26] |
Li, H.-Q.; Yang, J.; Ma, S.; Qiao, C. Bioorg. Med. Chem. 2012, 20, 4194.
doi: 10.1016/j.bmc.2012.05.079 |
| [27] |
Bai, S.-P.; Li, S.-H.; Xu, J.-B.; Peng, X.; Sai, K.; Chu, W.; Tu, Z.; Zeng, C.; Mach, R. H. J. Med. Chem. 2014, 57, 4239.
doi: 10.1021/jm5001453 |
| [28] |
Bertrand, H. C.; Schaap, M.; Baird, L.; Georgakopoulos, N. D.; Fowkes, A.; Thiollier, C.; Kachi, H.; Dinkova-Kostova, A. T.; Wells, G. J. Med. Chem. 2015, 58, 7186.
doi: 10.1021/acs.jmedchem.5b00602 pmid: 26348784 |
| [29] |
Manandhar, E.; Broome, J. H.; Myrick, J.; Lagrone, W.; Cragg, P. J.; Wallace, K. J. Chem. Commun. 2011, 47, 8796.
doi: 10.1039/c1cc13286e |
| [30] |
Colombano, G.; Albani, C.; Ottonello, G.; Ribeiro, A.; Scarpelli, R.; Tarozzo, G.; Daglian, J.; Jung, K.-M.; Piomelli, D.; Bandiera, T. ChemMedChem 2015, 10, 380.
doi: 10.1002/cmdc.201402374 |
| [31] |
Sánchez-Sixto, C.; Prazeres, V. F. V.; Castedo, L.; Suh, S. W.; Lamb, H.; Hawkins, A. R.; Cañada, F. J.; Jiménez-Barbero, J.; González-Bello, C. ChemMedChem 2008, 3, 756.
doi: 10.1002/cmdc.200700307 pmid: 18200648 |
| [32] |
Pirali, T.; Gatti, S.; Di Brisco, R.; Tacchi, S.; Zaninetti, R.; Brunelli, E.; Massarotti, A.; Sorba, G.; Canonico, P. L.; Moro, L.; Genazzani, A. A.; Tron, G. C.; Billington, R. A. ChemMedChem 2007, 2, 437.
doi: 10.1002/cmdc.200600192 |
| [33] |
Zhang, Q.; Takacs, J. M. Org. Lett. 2008, 10, 545.
doi: 10.1021/ol702890s pmid: 18189407 |
| [34] |
Park, K. D.; Morieux, P.; Salomé, C.; Cotten, S. W.; Reamtong, O.; Eyers, C.; Gaskell, S. J.; Stables, J. P.; Liu, R.; Kohn, H. J. Med. Chem. 2009, 52, 6897.
doi: 10.1021/jm9012054 pmid: 19795888 |
| [1] | Zeren Sun, Bingxin Zhai, Guangchao He, Hui Shen, Linya Chen, Shan Zhang, Yi Zou, Qihua Zhu, Yungen Xu. Synthesis and Anti-inflammatory Evaluation of Novel 1,2,3-Triazole Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2143-2155. |
| [2] | Jian Ji, Jinhua Liu, Cong Guan, Xuwen Chen, Yun Zhao, Shunying Liu. High Regioselective Synthesis of N2-Substituted-1,2,3-triazole via N-Sulfonyl-1,2,3-triazole Coupling with Alcohol Catalyzed by in-situ Generated Sulfonic Acid [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1168-1176. |
| [3] | Zujia Lu, Jian Qin, Jinting Wu, Wenli Cao, Baolong Kuang, Jianguo Zhang. Advances in the Synthesis of Energetic Compounds Based on 1,2,3-Triazoles [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 526-554. |
| [4] | Dongying Li, Shanguang Qiu, Yuxue Chen, Yanmei Zhao, Yunlong Wei, Luyong Wu, Wenhao Chen. Research for the Reaction of Base-Catalyzed Selective Hydrogen- Deuterium Exchange in Mono-1-substituted 1,2,3-Triazoles [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2898-2905. |
| [5] | Chao Gao, Xiaojie Si, Lingling Chi, Hao Wang, Honglin Dai, Limin Liu, Zhengjie Wang, Yang Zhang, Tao Wang, Yaochuan Zhou, Jiaxin Zheng, Yu Ke, Hongmin Liu, Qiurong Zhang. Synthesis and Antiproliferative Activity of 2,4,5,6-Tetrasubstituted Pyrimidine Derivatives Containing Anisole [J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1677-1686. |
| [6] | Xiu Wang, Wengui Duan, Guishan Lin, Baoyu Li, Wenjing Zhang, Fuhou Lei. Synthesis, Antifungal Activity, Three-Dimensional Quantitative Structure-Activity Relationship and Molecular Docking Study of 4-Acyl-3-amino-1,2,4-triazole-thioether Derivatives Containing Natural Pinene Structure [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 871-883. |
| [7] | Qiang Huang, Tingting Deng, Jiayun Zhu, Jun Li, Feifei Li. Study on the Green Synthesis of β-Hydroxy-1,2,3-triazoles Catalyzed by An Amino-Functionalized Graphene-Supported Ag-Cu Composites [J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 534-542. |
| [8] | Yaquan Cao, Yingxue Yang, Hongjin Zhai, Jin Wang, Shuo Zhang, Huanhuan Wang, Pu Yang, Chunli Wu. Synthesis and Antitumor Activity of Novel 5- and 6-Substituted Indazole Derivatives [J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 590-599. |
| [9] | Chao Gao, Yutong Zhang, Lingling Chi, Hao Wang, Jiajie Ma, Mengxin Bi, Honglin Dai, Xiaojie Si, Limin Liu, Yang Zhang, Jiaxin Zheng, Yu Ke, Hongmin Liu, Qiurong Zhang. Synthesis and Antiproliferative Activity Evaluation of Novel 2,4,6-Trisubstituted Pyrimidine Derivatives [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3824-3834. |
| [10] | Xixi Zheng, Yunyun Liu, Jie-Ping Wan. Metal-Free Synthesis of 1,2,3-Triazoles in Pure Water via the Enamine Modified Annulation Reactions with Tosyl Azide [J]. Chinese Journal of Organic Chemistry, 2021, 41(7): 2700-2706. |
| [11] | Jingjing Bi, Xiaoxiao Sun, Song Gao, Changpo Chen, Guisheng Zhang. Copper Catalyzed Synthesis of 2H-1,2,3-Triazoles in Green Solvent [J]. Chinese Journal of Organic Chemistry, 2021, 41(7): 2760-2766. |
| [12] | Chengfei Li, Bo Cen, Wengui Duan, Guishan Lin, Xiu Wang, Baoyu Li. Synthesis, Herbicidal Activity and Three-Dimensional Quantitative Structure-Activity Relationship (3D-QSAR) Study of 4-Methyl- 1,2,4-triazole-thioether Compounds Containing Natural Styrene Structure [J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2485-2495. |
| [13] | Zhiheng Zhao, Ming Li, Yaqin Zhou, Yonghui He, Lizhu Zhang, Ganpeng Li, Lijun Gu. Synthesis of 1,2,4-Triazoles via the Electrochemical Oxidative [3+2] Annulation [J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2476-2484. |
| [14] | Tao Zhang, Nianxian Li, Nanqian Zhou, Wen Ma, Haiyuan Wei, Bingxin Zhang, Lianghui Chen, Guangfan Hai, Yingchao Duan, Suping Bai. Design, Synthesis and Biological Evaluation of Novel Thiazole-Fused Glaucocalyxin A Derivatives [J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2393-2400. |
| [15] | Yujia Sun, Ziwei Wang, Yan Wang, Tongxiu Xu, Keqing Tian, Ping Zhang. Synthesis and Antimicrobial Activity of 1,5-Benzothiazepines Incorporated with Triazole Moiety [J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2361-2373. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||