Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (12): 4220-4246.DOI: 10.6023/cjoc202206012 Previous Articles Next Articles
Special Issue: 自由基化学专辑
REVIEWS
收稿日期:2022-06-09
修回日期:2022-07-20
发布日期:2022-08-17
通讯作者:
何燕, 陈艳, 吴琼
Yan He( ), Tianzi Huang, Xiaoqin Shi, Yan Chen(
), Tianzi Huang, Xiaoqin Shi, Yan Chen( ), Qiong Wu(
), Qiong Wu( )
)
Received:2022-06-09
Revised:2022-07-20
Published:2022-08-17
Contact:
Yan He, Yan Chen, Qiong Wu
Share
Yan He, Tianzi Huang, Xiaoqin Shi, Yan Chen, Qiong Wu. Recent Advances in Photocatalytic Reactions with Isocyanides[J]. Chinese Journal of Organic Chemistry, 2022, 42(12): 4220-4246.
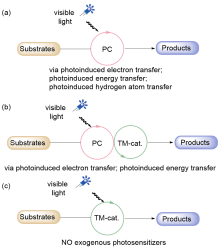
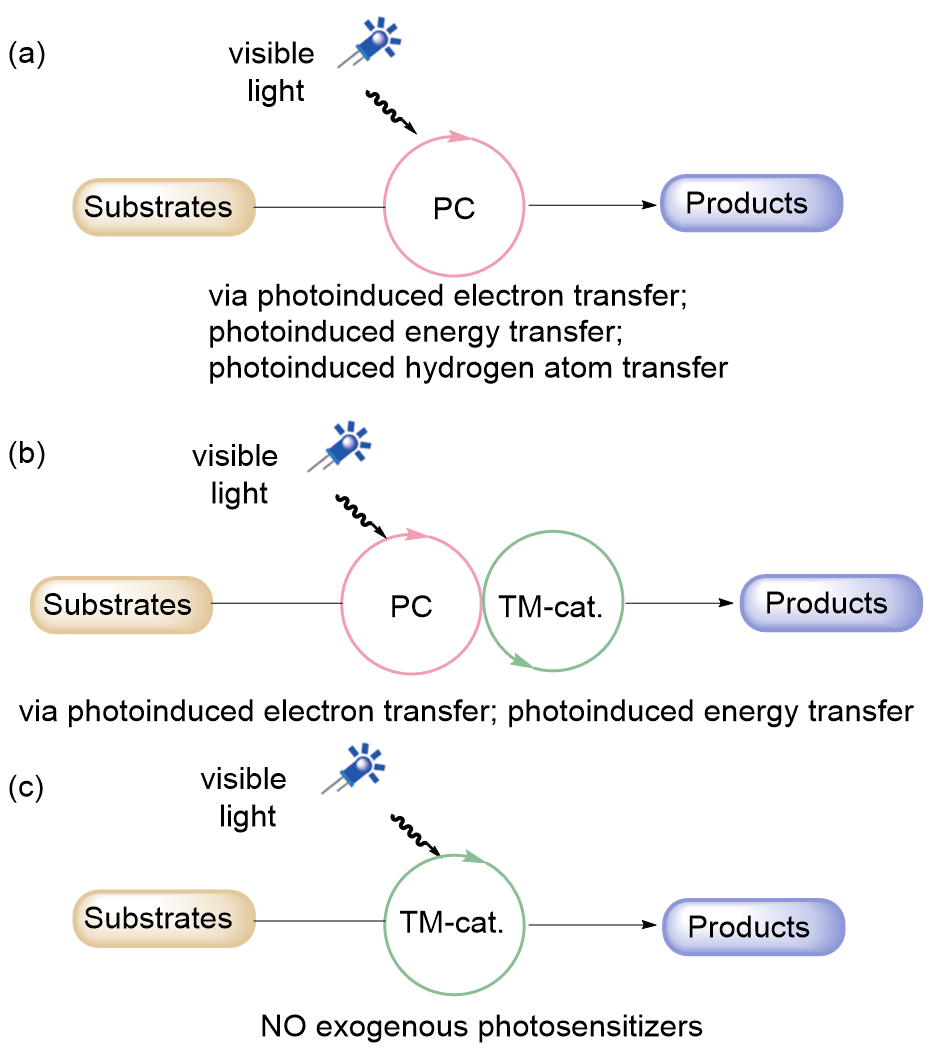
| Entry | Photocatalyst | E1/2(M+/M*)/V | E1/2(M*/M–)/V | E1/2(M+/M)/V | E1/2(M/M–)/V | Ref. |
|---|---|---|---|---|---|---|
| 1 | fac-Ir(ppy)3 | –1.73 | +0.31 | +0.77 | –2.19 | [13] |
| 2 | Ir[dF(CF3)ppy]2(dtb-bpy)+ | –0.89 | +1.21 | +1.69 | –1.37 | [14] |
| 3 | [Ir(ppy)2(dtbbpy)]+ | –0.96 | +0.66 | +1.21 | –1.51 | [14,15] |
| 4 | Ru(bpy)32+ | –0.81 | +0.77 | +1.29 | –1.33 | [16] |
| 5 | Cz-NI | –1.67 | +1.28 | +1.01 | –1.40 | [17] |
| 6 | MANI | –1.67 | +1.11 | +0.99 | –1.55 | [17] |
| 7 | Rose Bengal | –0.68 | +0.99 | +1.09 | –0.78 | [18] |
| 8 | Eosin Y | –1.60 | +1.18 | +0.72 | –1.14 | [18] |
| Entry | Photocatalyst | E1/2(M+/M*)/V | E1/2(M*/M–)/V | E1/2(M+/M)/V | E1/2(M/M–)/V | Ref. |
|---|---|---|---|---|---|---|
| 1 | fac-Ir(ppy)3 | –1.73 | +0.31 | +0.77 | –2.19 | [13] |
| 2 | Ir[dF(CF3)ppy]2(dtb-bpy)+ | –0.89 | +1.21 | +1.69 | –1.37 | [14] |
| 3 | [Ir(ppy)2(dtbbpy)]+ | –0.96 | +0.66 | +1.21 | –1.51 | [14,15] |
| 4 | Ru(bpy)32+ | –0.81 | +0.77 | +1.29 | –1.33 | [16] |
| 5 | Cz-NI | –1.67 | +1.28 | +1.01 | –1.40 | [17] |
| 6 | MANI | –1.67 | +1.11 | +0.99 | –1.55 | [17] |
| 7 | Rose Bengal | –0.68 | +0.99 | +1.09 | –0.78 | [18] |
| 8 | Eosin Y | –1.60 | +1.18 | +0.72 | –1.14 | [18] |
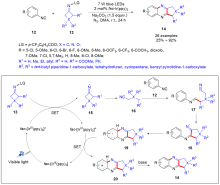
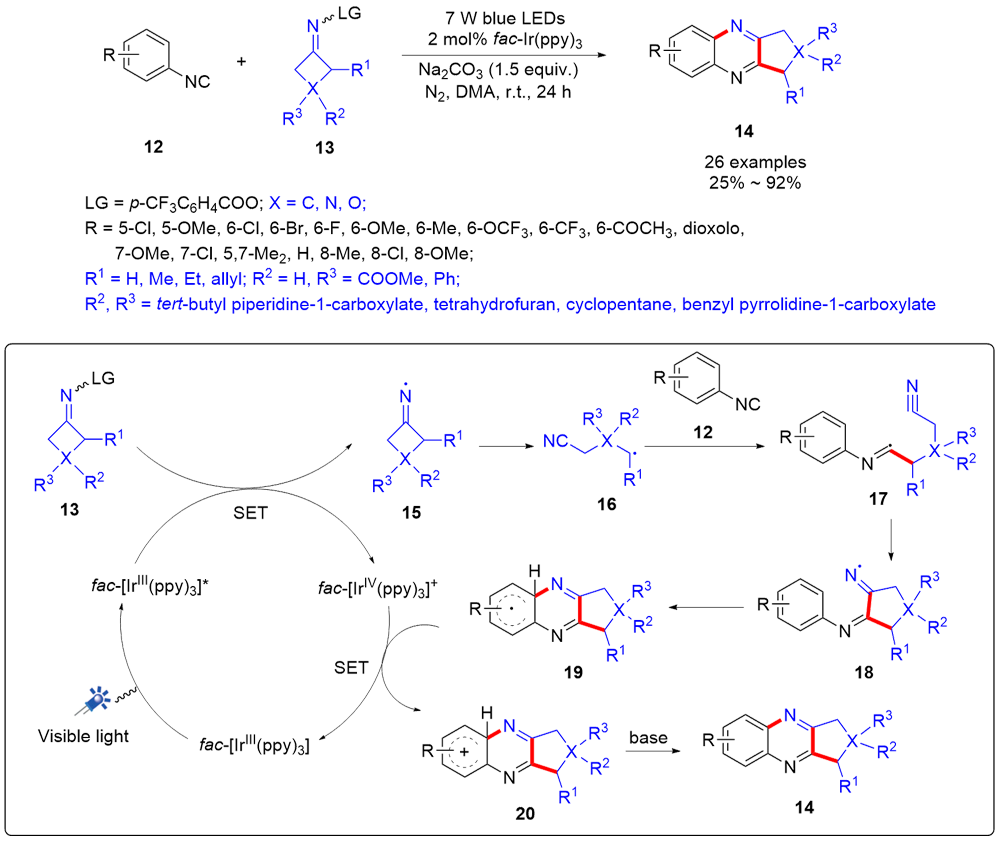
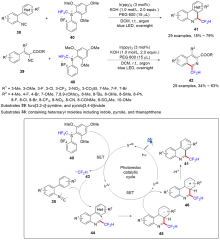

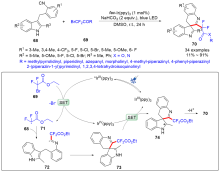

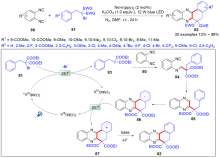
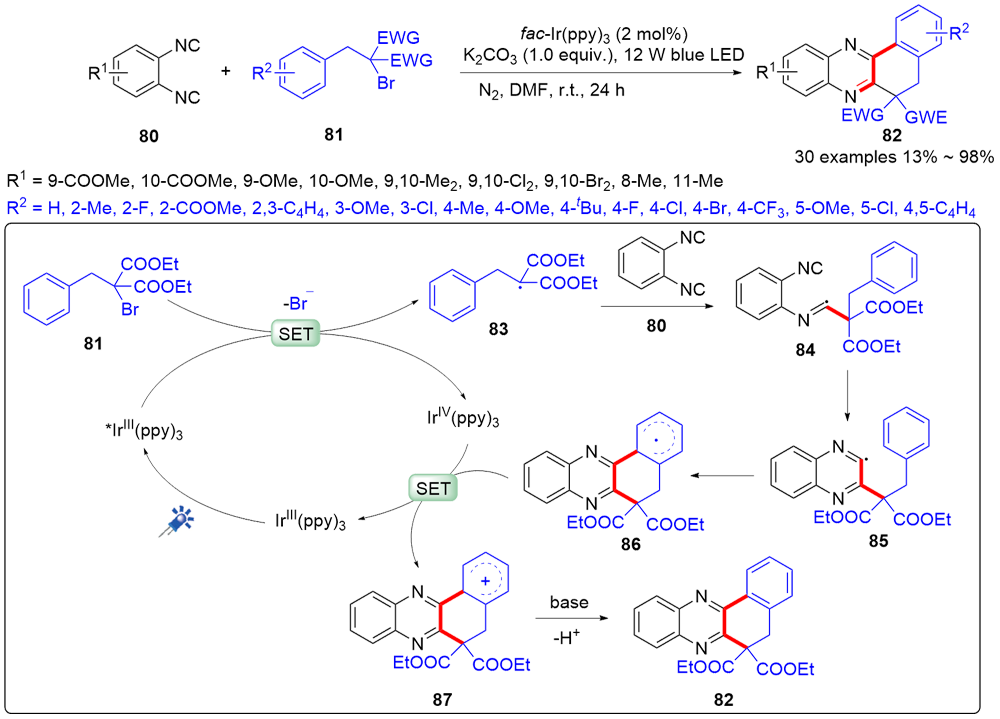

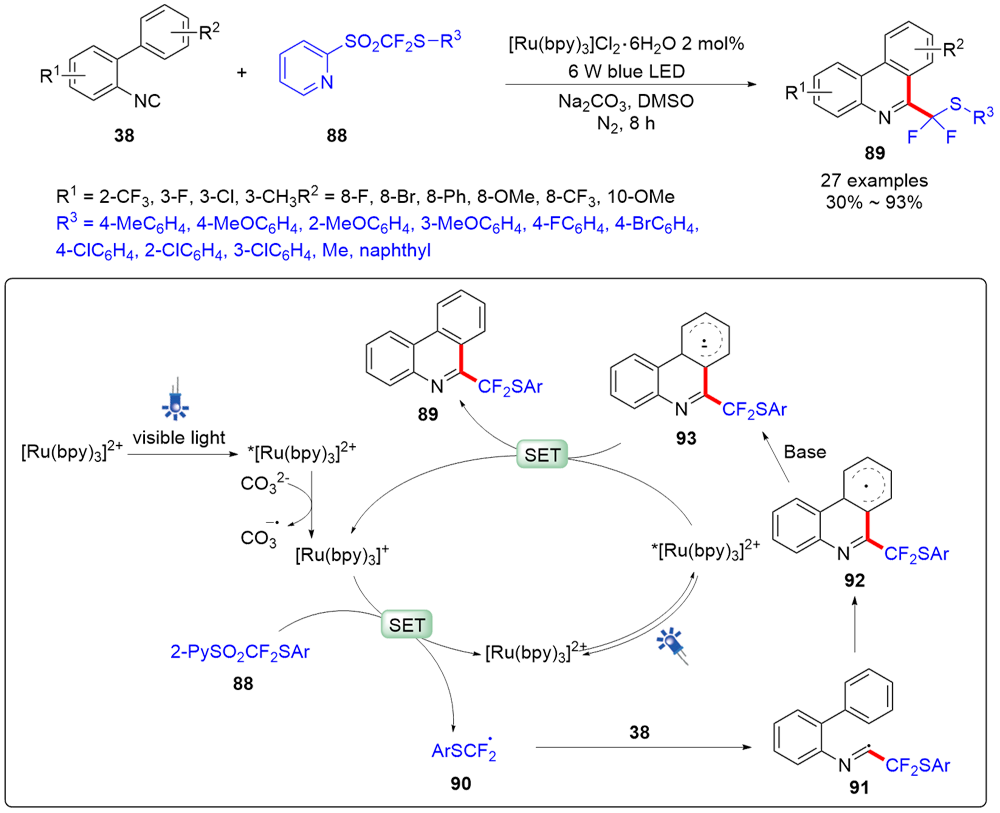
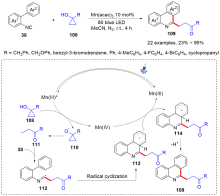

| [1] |
Schultz, D. M.; Yoon, T. P. Science 2014, 343, 985.
|
| [2] |
Ciamician, G. Science 1912, 36, 385.
pmid: 17836492 |
| [3] |
Cano-Yelo, H.; Deronzier, A. J. Chem. Soc., 1984, 1093.
|
| [4] |
König, B. Eur. J. Org. Chem. 2017, 1979.
|
| [5] |
Pandey, G.; Hajra, S.; Ghorai, M. K.; Kumar, K. R. J. Am. Chem. Soc. 1997, 119, 8777.
doi: 10.1021/ja9641564 |
| [6] |
Nicewicz, D. A.; MacMillan, D. W. C. Science 2008, 322, 77.
doi: 10.1126/science.1161976 pmid: 18772399 |
| [7] |
(a) Tucker, J. W.; Stephenson, C. R. J. J. Org. Chem. 2012, 77, 1617.
doi: 10.1021/jo202538x |
|
(b) Prier, C. K.; Rankic, D. A.; MacMillan, D. W. C. Chem. Rev. 2013, 113, 5322.
doi: 10.1021/cr300503r |
|
|
(c) Schultz, D. M.; Yoon, T. P. Science 2014, 343, 1239176.
doi: 10.1126/science.1239176 |
|
|
(d) Meggers, E. Chem. Commun. 2015, 51, 3290.
doi: 10.1039/C4CC09268F |
|
|
(e) Romero, N. A.; Nicewicz, D. A. Chem. Rev. 2016, 116, 10075.
doi: 10.1021/acs.chemrev.6b00057 |
|
| [8] |
(a) Tellis, J. C.; Kelly, C. B.; Primer, D. N.; Jouffroy, M.; Patel, N. R.; Molander, G. A. Acc. Chem. Res. 2016, 49, 1429.
doi: 10.1021/acs.accounts.6b00214 |
|
(b) Skubi, K. L.; Blum, T. R.; Yoon, T. P. Chem. Rev. 2016, 116, 10035.
doi: 10.1021/acs.chemrev.6b00018 |
|
|
(c) Lang, X.; Zhao, J.; Chen, X. Chem. Soc. Rev. 2016, 45, 3026.
doi: 10.1039/C5CS00659G |
|
| [9] |
(a) Nenaydenko, V. G. Isocyanide Chemistry: Applications in Synthesis and Material Science, Wiley-VCH, Weinheim, Germany, 2012.
pmid: 29620903 |
|
(b) Qiu, G.; Ding, Q.; Wu, J. Chem. Soc. Rev. 2013, 42, 5257.
doi: 10.1039/c3cs35507a pmid: 29620903 |
|
|
(c) Liu, J.; Fang, Z.; Zhang, Q.; Liu, Q.; Bi, X. Angew. Chem., Int. Ed. 2013, 52, 6953.
doi: 10.1002/anie.201302024 pmid: 29620903 |
|
|
(d) Rotstein, B. H.; Zaretsky, S.; Rai, V.; Yudin, A. K. Chem. Rev. 2014, 114, 8323.
doi: 10.1021/cr400615v pmid: 29620903 |
|
|
(e) He, Y.; Wang, Y.-C.; Hu, K.; Xu, X.-L.; Wang, H.-S.; Pan, Y.-M. J. Org. Chem. 2016, 81, 11813.
doi: 10.1021/acs.joc.6b02288 pmid: 29620903 |
|
|
(f) Song, B.; Xu, B. Chem. Soc. Rev. 2017, 46, 1103.
doi: 10.1039/C6CS00384B pmid: 29620903 |
|
|
(g) Giustiniano, M.; Basso, A.; Mercalli, V.; Massarotti, A.; Novellino, E.; Tron, G. C.; Zhu, J. Chem. Soc. Rev. 2017, 46, 1295.
doi: 10.1039/C6CS00444J pmid: 29620903 |
|
|
(h) Zhang, R.; Gu, Z.-Y.; Wang, S.-Y.; Ji, S.-J. Org. Lett. 2018, 20, 5510.
doi: 10.1021/acs.orglett.8b02516 pmid: 29620903 |
|
|
(i) Wan, J.-P.; Gana, L.; Liu, Y. Org. Biomol. Chem. 2017, 15, 9031.
doi: 10.1039/C7OB02011B pmid: 29620903 |
|
|
(j) He, Y.; Wang, Y.; Liang, X.; Huang, B.; Wang, H.; Pan, Y.-M. Org. Lett. 2018, 20, 7117.
doi: 10.1021/acs.orglett.8b03068 pmid: 29620903 |
|
|
(k) Tong, W.; Li, W.-H.; He, Y.; Mo, Z.-Y.; Tang, H.-T.; Wang, H.-S.; Pan, Y.-M. Org. Lett. 2018, 20, 2494.
doi: 10.1021/acs.orglett.8b00886 pmid: 29620903 |
|
|
(l) Cao, M.; Liu, L. Tang, S.; Peng, Z.; Wang, Y. Adv. Synth. Catal. 2019, 361, 1887.
doi: 10.1002/adsc.201801245 pmid: 29620903 |
|
|
(m) Wang, M.-R.; Deng, L.; Liu, G.-C.; Wen, L.; Wang, J.-G.; Huang, K.-B.; Tang, H.-T.; Pan, Y.-M. Org. Lett. 2019, 21, 4929.
doi: 10.1021/acs.orglett.9b01230 pmid: 29620903 |
|
|
(n) Cao, M.; Fang, Y.-L.; Wang, Y.-C.; Xu, X.-J.; Xi, Z.-W.; Tang, S. ACS Comb. Sci. 2020, 22, 268.
doi: 10.1021/acscombsci.0c00012 pmid: 29620903 |
|
|
(o) Cao, M.; Teng, Q.-H.; Xi, Z.-W.; Liu, L.-Q.; Gu, R.-Y.; Wang, Y.-C. Org. Biomol. Chem. 2020, 18, 655.
doi: 10.1039/C9OB02337B pmid: 29620903 |
|
|
(p) Xi, Z.-W.; He, Y.; Liu, L.-Q.; Wang, Y.-C. Org. Biomol. Chem. 2020, 18, 8089.
doi: 10.1039/D0OB01604G pmid: 29620903 |
|
|
(q) Li, Y.; Miao, T.; Li, P.; Wang, L. Org. Lett. 2018, 20, 1735.
doi: 10.1021/acs.orglett.8b00171 pmid: 29620903 |
|
| [10] |
Ren, X.; Lu, Z. Chin. J. Catal. 2019, 40, 1003.
|
| [11] |
Heitz, D. R.; Tellis, J. C.; Molander, G. A. J. Am. Chem. Soc. 2016, 138, 12715.
pmid: 27653500 |
| [12] |
Hoffmann, N. Eur. J. Org. Chem. 2017, 15, 1982.
|
| [13] |
Flamigni, L.; Barbieri, A.; Sabatini, C.; Ventura, B.; Barigelletti, F. Top. Curr. Chem. 2007, 281, 143.
|
| [14] |
Lowry, M. S.; Goldsmith, J. I.; Slinker, J. D.; Rohl, R.; Pascal, R. A.; Malliaras, G. G.; Bernhard, S. Chem. Mater. 2005, 17, 5712.
doi: 10.1021/cm051312+ |
| [15] |
Slinker, J. D.; Gorodetsky, A. A.; Lowry, M. S.; Wang, J.; Parker, S.; Rohl, R.; Bernhard, S.; Malliaras, G. G. J. Am. Chem. Soc. 2004, 126, 2763.
pmid: 14995193 |
| [16] |
(a) Kalyanasundaram, K. Coord. Chem. Rev. 1982, 46, 159.
doi: 10.1016/0010-8545(82)85003-0 |
|
(b) Juris, A.; Balzani, V.; Belser, P.; von Zelewsky, A. Helv. Chim. Acta 1981, 64, 2175.
doi: 10.1002/hlca.19810640723 |
|
| [17] |
Yi, M.-J.; Zhang, H.-X.; Xiao, T.-F.; Zhang, J.-H.; Feng, Z.-T.; Wei, L.-P.; Xu, G.-Q.; Xu, P.-F. ACS Catal. 2021, 11, 3466.
doi: 10.1021/acscatal.1c00242 |
| [18] |
Ravelli, D.; Fagnonia, M.; Albini, A. Chem. Soc. Rev. 2013, 42, 97.
doi: 10.1039/c2cs35250h pmid: 22990664 |
| [19] |
Vidyasagar, A.; Shi, J.; Kreitmeier, P.; Reiser, O. Org. Lett. 2018, 20, 6984.
doi: 10.1021/acs.orglett.8b02725 pmid: 30398887 |
| [20] |
Yuan, Y.; Dong, W.-H.; Gao, X.-S.; Xie, X.-M.; Zhang, Z.-G.; Chem. Commun. 2019, 55, 11900.
doi: 10.1039/C9CC05655F |
| [21] |
Yuan, Y.; Dong, W.; Gao, X.; Xie, X.; Zhang, Z. Org. Lett. 2019, 21, 469.
doi: 10.1021/acs.orglett.8b03710 pmid: 30588818 |
| [22] |
Zhang, X.; Zhu, P.; Zhang, R.; Li, X.; Yao, T. J. Org. Chem. 2020, 85, 9503.
doi: 10.1021/acs.joc.0c00039 |
| [23] |
Qin, W.-B.; Xiong, W.; Li, X.; Chen, J.-Y.; Lin, L.-T.; Wong, H. N. C.; Liu, G.-K. J. Org. Chem. 2020, 85, 10479.
doi: 10.1021/acs.joc.0c00816 |
| [24] |
Cannalire, R.; Amato, J.; Summa, V.; Novellino, E.; Tron, G. C.; Giustiniano, M. J. Org. Chem. 2020, 85, 14077.
doi: 10.1021/acs.joc.0c01946 pmid: 33074674 |
| [25] |
Pelliccia, S.; Alfano, A. I.; Luciano, P.; Novellino, E.; Massarotti, A.; Tron, G. C.; Ravelli, D.; Giustiniano, M. J. Org. Chem. 2020, 85, 1981.
doi: 10.1021/acs.joc.9b02709 pmid: 31880934 |
| [26] |
Cannalire, R.; Santoro, F.; Russo, C.; Graziani, G.; Tron, G. C.; Carotenuto, A.; Brancaccio, D.; Giustiniano, M. ACS Org. Inorg. Au 2022, 2, 66.
doi: 10.1021/acsorginorgau.1c00028 |
| [27] |
Zhang, J.; Xu, W.; Qu, Y.; Liu, Y.; Li, Y.; Song, H.; Wang, Q.; Chem. Commun. 2020, 56, 15212.
doi: 10.1039/D0CC06645A |
| [28] |
López-Mendoza, P.; Miranda, L. D. Org. Biomol. Chem. 2020, 18, 3487.
doi: 10.1039/d0ob00136h pmid: 32347280 |
| [29] |
Yuan, Y.; Zhang, S.-Y.; Dong, W.-H.; Wu, F.; Xie, X.-M.; Zhang, Z.-G. Adv. Synth. Catal. 2021, 363, 4216.
doi: 10.1002/adsc.202100474 |
| [30] |
Wei, J.; Gu, D.; Wang, S.; Hu, J.; Dong, X.; Sheng, R. Org. Chem. Front. 2018, 5, 2568.
doi: 10.1039/C8QO00644J |
| [31] |
Yuan, Y.; Dong, W.; Gao, X.; Gao, H.; Xie, X.; Zhang, Z. J. Org. Chem. 2018, 83, 2840.
doi: 10.1021/acs.joc.7b03283 pmid: 29411608 |
| [32] |
Zhu, Z.-F.; Zhang, M.-M.; Liu, F. Org. Biomol. Chem. 2019, 17, 1531.
doi: 10.1039/C8OB02786B |
| [33] |
(a) Liu, W.; Ackermann, L. ACS Catal. 2016, 6, 3743.
doi: 10.1021/acscatal.6b00993 |
|
(b) Mukherjee, A.; Milstein, D. ACS Catal. 2018, 8, 11435.
doi: 10.1021/acscatal.8b02869 |
|
|
(c) Rohit, K. R.; Radhika, S.; Saranya, S.; Anilkumar, G. Adv. Synth. Catal. 2020, 362, 1602.
doi: 10.1002/adsc.201901389 |
|
|
(d) Cano, R.; Mackey, K.; McGlacken, G. P. Catal. Sci. Technol. 2018, 8, 1251.
doi: 10.1039/C7CY02514A |
|
|
(e) Das, K.; Mondal, A.; Pal, D.; Srivastava, H. K.; Srimani, D. Organometallics 2019, 38, 1815.
doi: 10.1021/acs.organomet.9b00131 |
|
|
(f) Das, K.; Mondal, A.; Srimani, D. Chem. Commun. 2018, 54, 10582.
doi: 10.1039/C8CC05877F |
|
|
(g) Das, K.; Mondal, A.; Srimani, D. J. Org. Chem. 2018, 83, 9553.
doi: 10.1021/acs.joc.8b01316 |
|
| [34] |
Cheng, W.-M.; Shang, R. ACS Catal. 2020, 10, 9170.
doi: 10.1021/acscatal.0c01979 |
| [35] |
Wang, L.; Ding, Q.; Li, X.; Peng, Y. Asian J. Org. Chem. 2019, 8, 385.
doi: 10.1002/ajoc.201800733 |
| [36] |
Wei, W.; Bao, P.; Yue, H.; Liu, S.; Wang, L.; Li, Y.; Yang, D. Org. Lett. 2018, 20, 5291.
doi: 10.1021/acs.orglett.8b02231 pmid: 30129769 |
| [37] |
Singh, M.; Yadav, A. K.; Yadav, L. D. S.; Singh, R. K. P. Synlett 2018, 29, 176.
doi: 10.1055/s-0036-1590921 |
| [38] |
Lv, Y.; Bao, P.; Yue, H.; Li, J.-S.; Wei, W. Green Chem. 2019, 21, 6051.
doi: 10.1039/C9GC03253C |
| [39] |
Yang, W.; Li, B.; Zhang, M.; Wang, S.; Ji, Y.; Dong, S.; Feng, J.; Yuan, S. Chin. Chem. Lett. 2020, 31, 1313.
doi: 10.1016/j.cclet.2019.10.022 |
| [40] |
Singh, H. K.; Kamal, A.; Kumari, S.; Maury, S. K.; Kushwaha, A. K.; Srivastava, V.; Singh, S. ChemistrySelect 2021, 6, 13982.
doi: 10.1002/slct.202103548 |
| [41] |
Dahiya, A.; Das, B.; Sahoo, A. K.; Patel, B. K. Adv. Synth. Catal. 2022, 364, 966.
doi: 10.1002/adsc.202101431 |
| [42] |
Liu, Y.; Kang, Y.; Wang, H.; Dong, Y.; Yang, T.; Wei, Q.; Jiang, Y.; Yang, Y.; Gao, H. Tetrahedron Lett. 2020, 61, 152348.
doi: 10.1016/j.tetlet.2020.152348 |
| [43] |
(a) Jones, G. H.; Edwards, D. W.; Parr, D. A. J. Chem. Soc., Chem. Commun. 1976, 969.
|
|
(b) West, J. G.; Huang, D.; Sorensen, E. J. Nat. Commun. 2015, 6, 10093.
doi: 10.1038/ncomms10093 |
|
| [44] |
Singh, H. K.; Kamal, A.; Kumari, S.; Kumar, D.; Maury, S. K. Srivastava, V.; Singh, S. ACS Omega 2020, 5, 29854.
doi: 10.1021/acsomega.0c03941 |
| [45] |
Santos, M. S.; Betim, H. L. I.; Kisukuri, C. M.; Delgado, J. A. C.; Corrêa, A. G.; Paixão, M. W. Org. Lett. 2020, 22, 4266.
doi: 10.1021/acs.orglett.0c01297 |
| [46] |
(a) Zhou, Z.; Kong, X.; Liu, T. Chin. J. Org. Chem. 2021, 41, 3844. (in Chinese)
doi: 10.6023/cjoc202106001 |
|
( 周子杰, 孔祥梅, 刘天飞, 有机化学, 2021, 41, 3844.)
|
|
|
(b) Gentry, E. C.; Knowles, R. R. Acc. Chem. Res. 2016, 49, 1546.
doi: 10.1021/acs.accounts.6b00272 |
|
| [47] |
Liu, Y.; Chen, X.-L.; Li, X.-Y.; Zhu, S.-S.; Li, S.-J.; Song, Y.; Qu, L.-B.; Yu, B. J. Am. Chem. Soc. 2021, 143, 964.
doi: 10.1021/jacs.0c11138 |
| [48] |
Gore, B. S.; Kuo, C.-Y.; Wang, J.-J. Chem. Commun. 2022, 58, 4087.
doi: 10.1039/D2CC00717G |
| [49] |
Cheung, K. P. S.; Sarkar, S.; Gevorgyan, V. Chem. Rev. 2022, 122, 1543.
doi: 10.1021/acs.chemrev.1c00403 |
| [50] |
(a) Kunkely, H.; Vogler, A. J. Organomet. Chem. 1998, 559, 215.
doi: 10.1016/S0022-328X(98)00411-2 |
|
(b) Caspar, J. V. J. Am. Chem. Soc. 1985, 107, 6718.
doi: 10.1021/ja00309a055 |
|
|
(c) Harvey, P. D.; Gray, H. B. J. Am. Chem. Soc. 1988, 110, 2145.
doi: 10.1021/ja00215a023 |
|
|
(d) Harvey, P. D.; Schaefer, W. P.; Gray, H. B. Inorg. Chem. 1988, 27, 1101.
doi: 10.1021/ic00279a034 |
|
|
(e) Ohkubo, T.; Takao, K.; Tsubomura, T. Inorg. Chem. Commun. 2012, 20, 27.
|
|
|
(f) Riese, S.; Holzapfel, M.; Schmiedel, A.; Gert, I.; Schmidt, D.; Wurthner, F.; Lambert, C. Inorg. Chem. 2018, 57, 12480.
doi: 10.1021/acs.inorgchem.8b00974 |
|
| [51] |
Parasram, M.; Chuentragool, P.; Sarkar, D.; Gevorgyan, V. J. Am. Chem. Soc. 2016, 138, 6340.
doi: 10.1021/jacs.6b01628 pmid: 27149524 |
| [52] |
Jia, X.; Zhang, Z.; Gevorgyan, V. ACS Catal. 2021, 11, 13217.
doi: 10.1021/acscatal.1c04183 |
| [1] | Yanshuo Zhu, Hongyan Wang, Penghua Shu, Ke'na Zhang, Qilin Wang. Recent Advances on Alkoxy Radicals-Mediated C(sp3)—H Bond Functionalization via 1,5-Hydrogen Atom Transfer [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 1-17. |
| [2] | Yingke Feng, He Wang, Mengxing Cui, Ran Sun, Xin Wang, Yang Chen, Lei Li. Visible-Light-Induced Difluoroalkylated Cyclization of Novel Functionalized Aromatic Isocyanides [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2913-2925. |
| [3] | Xiaona Yang, Hongyu Guo, Rong Zhou. Progress in Visible-Light Promoted Transformations of Organosilicon Compounds [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2720-2742. |
| [4] | Qiushan Gao, Meng Li, Wanqing Wu. Recent Advances in Transition Metal-Catalyzed Isocyanide Insertion Reactions [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2659-2681. |
| [5] | Haoyang Liu, Shuangshuang Sun, Xianli Ma, Yanyan Chen, Yanli Xu. Synthesis of Selenylated Spiro[indole-3,3'-quinoline] Derivatives via Visible-Light-Promoted Isocyanide Insertion [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2867-2876. |
| [6] | Xianghao Luo, Yibi Xie, Nianyu Huang, Long Wang. Ugi Four-Component Reaction Based on in-situ Capture of Isocyanide and Post-Modification Tandem Reaction: One-Pot Synthesis of Nitrogen Heterocycles [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 838-846. |
| [7] | Long Zhao, Maolin Yang, Haoran Chen, Mingwu Ding. One-Pot Three-Component Synthesis of 3,4-Dihydroquinazoline Derivatives [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3740-3746. |
| [8] | Xiuliang Cheng, Dong Li, Boxuan Yang, Yumei Lin, Lei Gong. Recent Advances in Visible-Light Photocatalytic Asymmetric Synthesis Enabled by Chiral Lewis Acids [J]. Chinese Journal of Organic Chemistry, 2022, 42(10): 3335-3350. |
| [9] | Weiqing Ma, Ying Han, Jin Sun, Chaoguo Yan. Three-Component Reaction for Efficient Synthesis of Functionalized Spiro[cyclopentane-1,3'-indolines] [J]. Chinese Journal of Organic Chemistry, 2021, 41(8): 3180-3191. |
| [10] | Yangyang Weng, Jingping Qu, Yifeng Chen. Palladium-Catalyzed Allylic Carbonylative Negishi Cross-Coupling Reactions with Sterically Bulky Aromatic Isocyanides [J]. Chinese Journal of Organic Chemistry, 2021, 41(5): 1949-1956. |
| [11] | Hongyu Wu, Xianyong Yu, Zhong Cao. Electrochemical Multicomponent Synthesis of α-Ketoamides from α-Oxocarboxylic Acids, Isocyanides and Water [J]. Chinese Journal of Organic Chemistry, 2021, 41(12): 4712-4717. |
| [12] | Chen Yuefeng, Zhao He, Cheng Dongping, Li Xiaonian, Xu Xiaoliang. Coupling of Hantzsch Esters with Baylis-Hillman Derivatives via Visible-Light Photoredox Catalysis [J]. Chinese Journal of Organic Chemistry, 2020, 40(5): 1297-1304. |
| [13] | Zhang Junhui, Niu Lizhi, Li Ying, Liu Si, Jiang Lin. Facile Synthesis of 2-(Pyridin-3-yl)-2-benzoyloxy Acetamides via Passerini Reaction and Evaluation of Their Biological Activity [J]. Chin. J. Org. Chem., 2018, 38(7): 1842-1848. |
| [14] | Sun Xiaoyang, Yu Shouyun. Synthesis of Polysubstituted (Hetero)aromatic Compounds Using Visible-Light-Promoted Radical Triple Bond Insertions [J]. Chin. J. Org. Chem., 2016, 36(2): 239-247. |
| [15] | Wang Hao, Xu Bin. Recent Advances in Inert Bonds Activation with Isocyanides [J]. Chin. J. Org. Chem., 2015, 35(3): 588-602. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||