Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (10): 3335-3350.DOI: 10.6023/cjoc202205032 Previous Articles Next Articles
Special Issue: 不对称催化专辑
REVIEWS
成秀亮a, 李冬a, 杨博轩a, 林玉妹a, 龚磊a,b,*( )
)
收稿日期:2022-05-19
修回日期:2022-06-25
发布日期:2022-11-02
通讯作者:
龚磊
作者简介:基金资助:
Xiuliang Chenga, Dong Lia, Boxuan Yanga, Yumei Lina, Lei Gonga,b( )
)
Received:2022-05-19
Revised:2022-06-25
Published:2022-11-02
Contact:
Lei Gong
About author:Supported by:Share
Xiuliang Cheng, Dong Li, Boxuan Yang, Yumei Lin, Lei Gong. Recent Advances in Visible-Light Photocatalytic Asymmetric Synthesis Enabled by Chiral Lewis Acids[J]. Chinese Journal of Organic Chemistry, 2022, 42(10): 3335-3350.

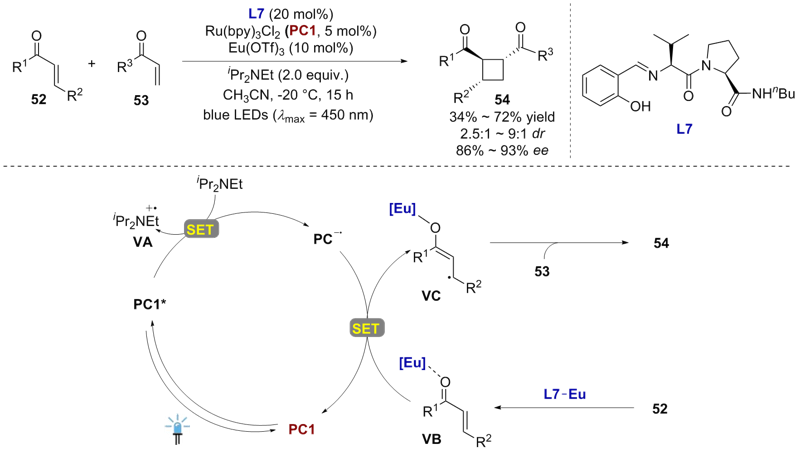


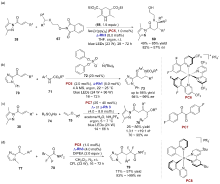
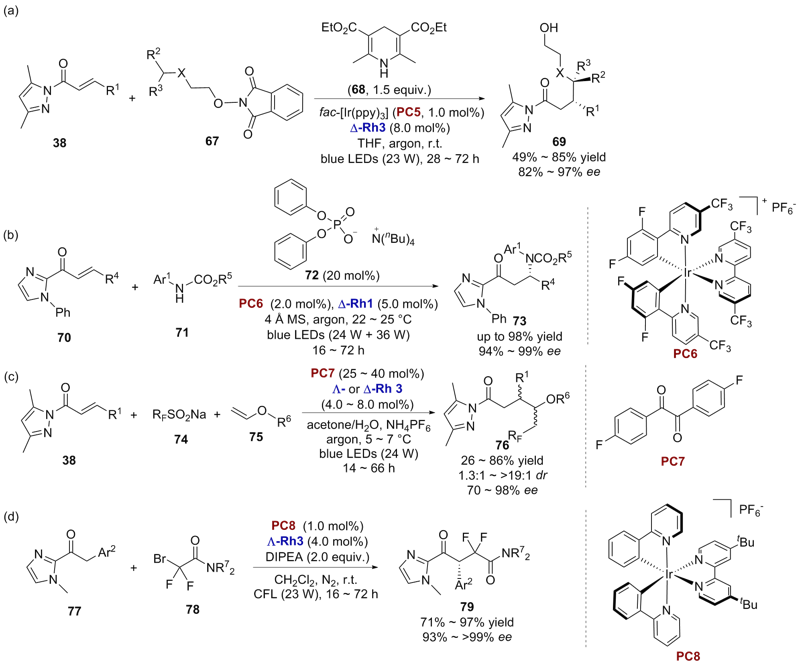
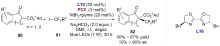


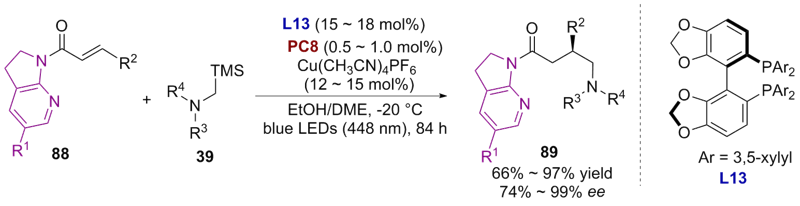
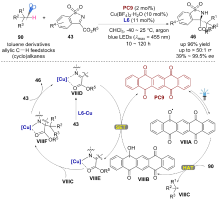


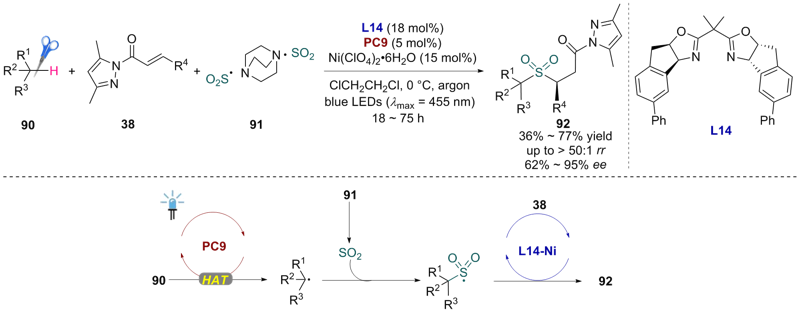
| [1] |
Prier, C. K.; Rankic, D. A.; MacMillan, D. W. Chem. Rev. 2013, 113, 5322.
doi: 10.1021/cr300503r |
| [2] |
Yoon, T. P.; Ischay, M. A.; Du, J. Nat. Chem. 2010, 2, 527.
doi: 10.1038/nchem.687 |
| [3] |
Zhang, H.-T.; Gu, L.-J.; Huang, X.-Z.; Wang, R.; Jin, C.; Li, G.-P. Chin. Chem. Lett. 2016, 27, 256.
doi: 10.1016/j.cclet.2015.10.012 |
| [4] |
Qu, Z.; Wang, P.; Chen, X.; Deng, G.-J.; Huang, H. Chin. Chem. Lett. 2021, 32, 2582.
doi: 10.1016/j.cclet.2021.02.047 |
| [5] |
Chen, J. R.; Hu, X. Q.; Lu, L. Q.; Xiao, W. J. Chem. Soc. Rev. 2016, 45, 2044.
doi: 10.1039/C5CS00655D |
| [6] |
Poplata, S.; Troster, A.; Zou, Y. Q.; Bach, T. Chem. Rev. 2016, 116, 9748.
doi: 10.1021/acs.chemrev.5b00723 pmid: 27018601 |
| [7] |
Zhou, Q. Q.; Zou, Y. Q.; Lu, L. Q.; Xiao, W. J. Angew. Chem., Int. Ed. 2019, 58, 1586.
doi: 10.1002/anie.201803102 |
| [8] |
Lipp, A.; Badir, S. O.; Molander, G. A. Angew. Chem., Int. Ed. 2021, 60, 1714.
doi: 10.1002/anie.202007668 |
| [9] |
Wang, C.; Lu, Z. Org. Chem. Front. 2015, 2, 179.
doi: 10.1039/C4QO00306C |
| [10] |
Meggers, E. Angew. Chem., Int. Ed. 2017, 56, 5668.
doi: 10.1002/anie.201612516 |
| [11] |
Vega-Peñaloza, A.; Paria, S.; Bonchio, M.; Dell’Amico, L.; Companyó, X. ACS Catal. 2019, 9, 6058.
doi: 10.1021/acscatal.9b01556 |
| [12] |
Jiang, C.; Chen, W.; Zheng, W. H.; Lu, H. Org. Biomol. Chem. 2019, 17, 8673.
doi: 10.1039/C9OB01609K |
| [13] |
Schwinger, D. P.; Bach, T. Acc. Chem. Res. 2020, 53, 1933.
doi: 10.1021/acs.accounts.0c00379 |
| [14] |
Li, S.; Xiang, S. H.; Tan, B. Chin. J. Chem. 2020, 38, 213.
doi: 10.1002/cjoc.201900472 |
| [15] |
Wang, A.-E.; Huang, P.-Q. Sci. China: Chem. 2020, 63, 871.
|
| [16] |
Santelli, M.; Pons, J. M. Lewis Acids and Selectivity in Organic Synthesis, CRC Press, Boca Raton, 1996.
|
| [17] |
Fukuzumi, S.; Jung, J.; Lee, Y. M.; Nam, W. Asian J. Org. Chem. 2017, 6, 397.
doi: 10.1002/ajoc.201600576 |
| [18] |
Yoon, T. P. Acc. Chem. Res. 2016, 49, 2307.
doi: 10.1021/acs.accounts.6b00280 |
| [19] |
Brimioulle, R.; Lenhart, D.; Maturi, M. M.; Bach, T. Angew. Chem., Int. Ed. 2015, 54, 3872.
doi: 10.1002/anie.201411409 |
| [20] |
Hong, B. C. Org. Biomol. Chem. 2020, 18, 4298.
doi: 10.1039/D0OB00759E |
| [21] |
Yang, K.; Song, Q. Acc. Chem. Res. 2021, 54, 2298.
doi: 10.1021/acs.accounts.1c00132 |
| [22] |
Lewis, F. D.; Howard, D. K.; Oxman, J. D. J. Am. Chem. Soc. 1983, 105, 3344.
doi: 10.1021/ja00348a069 |
| [23] |
Stegbauer, S.; Jandl, C.; Bach, T. Angew. Chem., Int. Ed. 2018, 57, 14593.
doi: 10.1002/anie.201808919 |
| [24] |
Leverenz, M.; Merten, C.; Dreuw, A.; Bach, T. J. Am. Chem. Soc. 2019, 141, 20053.
doi: 10.1021/jacs.9b12068 pmid: 31814393 |
| [25] |
Miyasaka, H. Acc. Chem. Res. 2013, 46, 248.
doi: 10.1021/ar300102t |
| [26] |
Crisenza, G. E. M.; Mazzarella, D.; Melchiorre, P. J. Am. Chem. Soc. 2020, 142, 5461.
doi: 10.1021/jacs.0c01416 pmid: 32134647 |
| [27] |
Kim, J. Y.; Lee, Y. S.; Ryu, D. H. ACS Catal. 2021, 11, 14811.
doi: 10.1021/acscatal.1c04835 |
| [28] |
Gong, L.; Wenzel, M.; Meggers, E. Acc. Chem. Res. 2013, 46, 2635.
doi: 10.1021/ar400083u |
| [29] |
Huang, X.; Meggers, E. Acc. Chem. Res. 2019, 52, 833.
doi: 10.1021/acs.accounts.9b00028 |
| [30] |
Huo, H.; Shen, X.; Wang, C.; Zhang, L.; Rose, P.; Chen, L. A.; Harms, K.; Marsch, M.; Hilt, G.; Meggers, E. Nature 2014, 515, 100.
doi: 10.1038/nature13892 |
| [31] |
Huo, H.; Wang, C.; Harms, K.; Meggers, E. J. Am. Chem. Soc. 2015, 137, 9551.
doi: 10.1021/jacs.5b06010 |
| [32] |
Wang, C.; Qin, J.; Shen, X.; Riedel, R.; Harms, K.; Meggers, E. Angew. Chem., Int. Ed. 2016, 55, 685.
doi: 10.1002/anie.201509524 |
| [33] |
Tan, Y.; Yuan, W.; Gong, L.; Meggers, E. Angew. Chem., Int. Ed. 2015, 54, 13045.
doi: 10.1002/anie.201506273 |
| [34] |
Shen, X.; Harms, K.; Marsch, M.; Meggers, E. Chem.-Eur. J. 2016, 22, 9102.
doi: 10.1002/chem.201601572 |
| [35] |
Steinlandt, P. S.; Zuo, W.; Harms, K.; Meggers, E. Chem.- Eur. J. 2019, 25, 15333.
doi: 10.1002/chem.201903369 |
| [36] |
Zhang, C.; Gao, A. Z.; Nie, X.; Ye, C. X.; Ivlev, S. I.; Chen, S.; Meggers, E. J. Am. Chem. Soc. 2021, 143, 13393.
doi: 10.1021/jacs.1c06637 |
| [37] |
Yoon, T. P. ACS Catal. 2013, 3, 895.
doi: 10.1021/cs400088e |
| [38] |
Huang, X.; Quinn, T. R.; Harms, K.; Webster, R. D.; Zhang, L.; Wiest, O.; Meggers, E. J. Am. Chem. Soc. 2017, 139, 9120.
doi: 10.1021/jacs.7b04363 |
| [39] |
Huang, X.; Li, X.; Xie, X.; Harms, K.; Riedel, R.; Meggers, E. Nat. Commun. 2017, 8, 2245.
doi: 10.1038/s41467-017-02148-1 |
| [40] |
Hu, N.; Jung, H.; Zheng, Y.; Lee, J.; Zhang, L.; Ullah, Z.; Xie, X.; Harms, K.; Baik, M. H.; Meggers, E. Angew. Chem., Int. Ed. 2018, 57, 6242.
doi: 10.1002/anie.201802891 |
| [41] |
Dai, X.; Li, Y.; Zhang, S.; Gong, L. Chin. J. Org. Chem. 2019, 39, 1711. (in Chinese).
doi: 10.6023/cjoc201902022 |
|
(代雪梅, 李延军, 张少南, 龚磊, 有机化学, 2019, 39, 1711.)
doi: 10.6023/cjoc201902022 |
|
| [42] |
Tasker, S. Z.; Standley, E. A.; Jamison, T. F. Nature 2014, 509, 299.
doi: 10.1038/nature13274 |
| [43] |
Zhang, S.; Cao, S.; Lin, Y.-M.; Sha, L.; Lu, C.; Gong, L. Chin. J. Catal. 2022, 43, 564.
doi: 10.1016/S1872-2067(21)63953-0 |
| [44] |
Tellis John, C.; Primer David, N.; Molander Gary, A. Science 2014, 345, 433.
doi: 10.1126/science.1253647 pmid: 24903560 |
| [45] |
Ding, W.; Lu, L. Q.; Zhou, Q. Q.; Wei, Y.; Chen, J. R.; Xiao, W. J. J. Am. Chem. Soc. 2017, 139, 63.
doi: 10.1021/jacs.6b11418 pmid: 28001382 |
| [46] |
Shen, X.; Li, Y.; Wen, Z.; Cao, S.; Hou, X.; Gong, L. Chem. Sci. 2018, 9, 4562.
doi: 10.1039/C8SC01219A |
| [47] |
Cao, S.; Ye, Z.; Chen, Y.; Lin, Y.-M.; Fang, J.; Wang, Y.; Yang, B.; Gong, L. CCS Chem. 2021, 3, 3329.
|
| [48] |
Li, Y.; Zhou, K.; Wen, Z.; Cao, S.; Shen, X.; Lei, M.; Gong, L. J. Am. Chem. Soc. 2018, 140, 15850.
doi: 10.1021/jacs.8b09251 |
| [49] |
Han, B.; Li, Y.; Yu, Y.; Gong, L. Nat. Commun. 2019, 10, 3804.
doi: 10.1038/s41467-019-11688-7 |
| [50] |
Zhou, K.; Yu, Y.; Lin, Y.-M.; Li, Y.; Gong, L. Green Chem. 2020, 22, 4597.
doi: 10.1039/D0GC00262C |
| [51] |
Skubi, K. L.; Blum, T. R.; Yoon, T. P. Chem. Rev. 2016, 116, 10035.
doi: 10.1021/acs.chemrev.6b00018 |
| [52] |
Du, J.; Skubi Kazimer, L.; Schultz Danielle, M.; Yoon Tehshik, P. Science 2014, 344, 392.
doi: 10.1126/science.1251511 |
| [53] |
Ruiz Espelt, L.; McPherson, I. S.; Wiensch, E. M.; Yoon, T. P. J. Am. Chem. Soc. 2015, 137, 2452.
doi: 10.1021/ja512746q pmid: 25668687 |
| [54] |
Dong, X.; Li, Q. Y.; Yoon, T. P. Org. Lett. 2021, 23, 5703.
doi: 10.1021/acs.orglett.1c01790 |
| [55] |
Amador, A. G.; Sherbrook, E. M.; Yoon, T. P. J. Am. Chem. Soc. 2016, 138, 4722.
doi: 10.1021/jacs.6b01728 |
| [56] |
Huang, X.; Webster, R. D.; Harms, K.; Meggers, E. J. Am. Chem. Soc. 2016, 138, 12636.
doi: 10.1021/jacs.6b07692 |
| [57] |
Huo, H.; Harms, K.; Meggers, E. J. Am. Chem. Soc. 2016, 138, 6936.
doi: 10.1021/jacs.6b03399 |
| [58] |
Wang, C.; Harms, K.; Meggers, E. Angew. Chem., Int. Ed. 2016, 55, 13495.
doi: 10.1002/anie.201607305 |
| [59] |
Zhou, Z.; Li, Y.; Han, B.; Gong, L.; Meggers, E. Chem. Sci. 2017, 8, 5757.
doi: 10.1039/C7SC02031G |
| [60] |
Ma, J.; Xie, X.; Meggers, E. Chem.-Eur. J. 2018, 24, 259.
doi: 10.1002/chem.201704619 |
| [61] |
Liang, H.; Xu, G. Q.; Feng, Z. T.; Wang, Z. Y.; Xu, P. F. J. Org. Chem. 2019, 84, 60.
doi: 10.1021/acs.joc.8b02316 pmid: 30507130 |
| [62] |
Liu, J.; Ding, W.; Zhou, Q. Q.; Liu, D.; Lu, L. Q.; Xiao, W. J. Org. Lett. 2018, 20, 461.
doi: 10.1021/acs.orglett.7b03826 |
| [63] |
Ye, C. X.; Melcamu, Y. Y.; Li, H. H.; Cheng, J. T.; Zhang, T. T.; Ruan, Y. P.; Zheng, X.; Lu, X.; Huang, P. Q. Nat. Commun. 2018, 9, 410.
doi: 10.1038/s41467-017-02698-4 pmid: 29379007 |
| [64] |
Zhang, K.; Lu, L. Q.; Jia, Y.; Wang, Y.; Lu, F. D.; Pan, F.; Xiao, W. J. Angew. Chem., Int. Ed. 2019, 58, 13375.
doi: 10.1002/anie.201907478 |
| [65] |
Pagire, S. K.; Kumagai, N.; Shibasaki, M. Chem. Sci. 2020, 11, 5168.
doi: 10.1039/D0SC01890B |
| [66] |
Labinger, J.; Bercaw, J. Nature 2002, 417, 507.
doi: 10.1038/417507a |
| [67] |
Goldberg, K. I.; Goldman, A. S. Acc. Chem. Res. 2017, 50, 620.
doi: 10.1021/acs.accounts.6b00621 |
| [68] |
Davies, H. M. L.; Liao, K. Nat. Rev. Chem. 2019, 3, 347.
doi: 10.1038/s41570-019-0099-x pmid: 32995499 |
| [69] |
Li, Y.; Lei, M.; Gong, L. Nat. Catal. 2019, 2, 1016.
doi: 10.1038/s41929-019-0357-9 |
| [70] |
Cao, S.; Hong, W.; Ye, Z.; Gong, L. Nat. Commun. 2021, 12, 2377.
doi: 10.1038/s41467-021-22690-3 |
| [1] | Huakun Wang, Xiaolong Ren, Yining Xuan. Study of the Halide Salt Catalyzed [3+2] Cycloaddition of α,β-Epoxy Carboxylate with Isocyanate [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 251-258. |
| [2] | Yanshuo Zhu, Hongyan Wang, Penghua Shu, Ke'na Zhang, Qilin Wang. Recent Advances on Alkoxy Radicals-Mediated C(sp3)—H Bond Functionalization via 1,5-Hydrogen Atom Transfer [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 1-17. |
| [3] | Shihang Yu, Jiawei Liu, Biyu An, Qinghua Bian, Min Wang, Jiangchun Zhong. Asymmetric Synthesis of the Contact Sex Pheromone of Neoclytus acuminatus acuminatus (Fabricius) [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 301-308. |
| [4] | Yuchao Wang, Jinbiao Liu, Zhitao He. Palladium-Catalyzed Asymmetric Hydrofunctionalizations of Conjugated Dienes [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2614-2627. |
| [5] | Xiaona Yang, Hongyu Guo, Rong Zhou. Progress in Visible-Light Promoted Transformations of Organosilicon Compounds [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2720-2742. |
| [6] | Tingyu Song, Ran Li, Lihua Huang, Shikun Jia, Guangjian Mei. Catalytic Asymmetric Synthesis of N—N Atropisomers [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 1977-1990. |
| [7] | Cheng Luo, Yanli Yin, Zhiyong Jiang. Recent Advances in Asymmetric Synthesis of P-Chiral Phosphine Oxides [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 1963-1976. |
| [8] | Ling Meng, Jun Wang. Research Progress on Synthesis of Thioflavonoids [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 873-891. |
| [9] | Huaiyuan Zhang, Nuo Xu, Rongping Tang, Xingli Shi. Recent Advances in Asymmetric Dearomatization Reactions Induced by Chiral Hypervalent Iodine Reagents [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3784-3805. |
| [10] | Liu-Yang Pu, Zhiyue Li, Limin Li, Yucui Ma, Min Ma, Shengquan Hu, Zhengzhi Wu. Asymmetric Synthesis of (–)-Colchicine and Its Natural Analog (–)-N-Acetylcolchicine Methyl Ether [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 313-319. |
| [11] | Yuanhao Mao, Yanfeng Gao, Zhiwei Miao. Research Progress on the Asymmetric Cyclization Synthesis of Seven-Membered Rings via Transition Metal Catalysis [J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 1904-1924. |
| [12] | Ting Yao, Jiayan Li, Jiaming Wang, Changgui Zhao. Recent Advances for the Construction of Seven-Membered Ring Catalyzed by N-Heterocyclic Carbenes [J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 925-944. |
| [13] | Lihua Wang, Xushun Gong, Ting Lei, Shizhi Jiang. Research Progress on Asymmetric Synthesis of Flavanones [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 758-769. |
| [14] | Yan He, Tianzi Huang, Xiaoqin Shi, Yan Chen, Qiong Wu. Recent Advances in Photocatalytic Reactions with Isocyanides [J]. Chinese Journal of Organic Chemistry, 2022, 42(12): 4220-4246. |
| [15] | Xiaoyuan Cui, Feng Zhou, Haihong Wu, Jian Zhou. Asymmetric Tandem Reactions Achieved by Chiral Amine & Gold(I) Cooperative Catalysis [J]. Chinese Journal of Organic Chemistry, 2022, 42(10): 3033-3050. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||