Chinese Journal of Organic Chemistry ›› 2023, Vol. 43 ›› Issue (5): 1761-1771.DOI: 10.6023/cjoc202302027 Previous Articles Next Articles
Special Issue: 有机硼化学专辑
ARTICLES
收稿日期:2023-02-25
修回日期:2023-05-14
发布日期:2023-05-15
通讯作者:
杨文, 赵万祥
基金资助:
Yuyuan Liu, Yaqin Lei, Wen Yang( ), Wanxiang Zhao(
), Wanxiang Zhao( )
)
Received:2023-02-25
Revised:2023-05-14
Published:2023-05-15
Contact:
Wen Yang, Wanxiang Zhao
Supported by:Share
Yuyuan Liu, Yaqin Lei, Wen Yang, Wanxiang Zhao. Cobalt-Catalyzed Remote Hydroboration of Enamines[J]. Chinese Journal of Organic Chemistry, 2023, 43(5): 1761-1771.
| Entry | Ligand | Solvent | Yield/% |
|---|---|---|---|
| 1 | Ph2PCy | THF | ND |
| 2 | PPh3 | THF | ND |
| 3 | L1 | THF | ND |
| 4 | L2 | THF | ND |
| 5 | L3 | THF | ND |
| 6 | L4 | THF | ND |
| 7 | L5 | THF | ND |
| 8 | L6 (tpy) | THF | 31 |
| 9 | L6 | DMF | ND |
| 10 | L6 | Cyclohexane | 57 |
| 11 | L6 | Octane | 74 |
| 12 | L6 | Toluene | 43 |
| 13 | L6 | CPME | 60 |
| 14 | L6 | DME | 54 |
| 15 | L6 | EA | 89 |
| Entry | Ligand | Solvent | Yield/% |
|---|---|---|---|
| 1 | Ph2PCy | THF | ND |
| 2 | PPh3 | THF | ND |
| 3 | L1 | THF | ND |
| 4 | L2 | THF | ND |
| 5 | L3 | THF | ND |
| 6 | L4 | THF | ND |
| 7 | L5 | THF | ND |
| 8 | L6 (tpy) | THF | 31 |
| 9 | L6 | DMF | ND |
| 10 | L6 | Cyclohexane | 57 |
| 11 | L6 | Octane | 74 |
| 12 | L6 | Toluene | 43 |
| 13 | L6 | CPME | 60 |
| 14 | L6 | DME | 54 |
| 15 | L6 | EA | 89 |
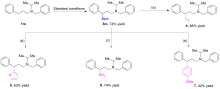
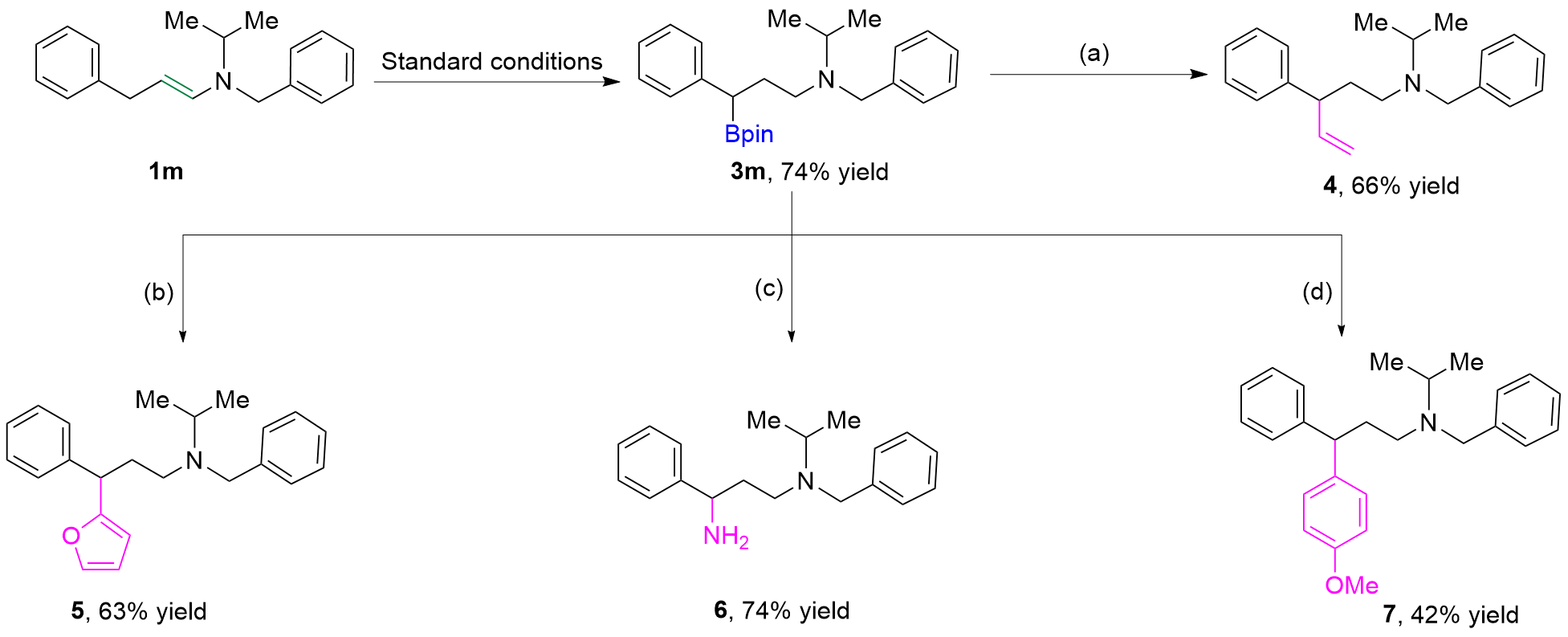
| [1] |
(a) Larionov, E.; Li, H.; Mazet, C. Chem. Commun. 2014, 50, 9816.
doi: 10.1039/C4CC02399D |
|
(b) Vasseur, A.; Bruffaerts, J.; Marek, I. Nat. Chem. 2016, 8, 209.
doi: 10.1038/nchem.2445 |
|
|
(c) Kochi, T.; Kanno, S.; Kakiuchi, F. Tetrahedron Lett. 2019, 60, 150938.
doi: 10.1016/j.tetlet.2019.07.029 |
|
|
(d) Janssen-Müller, D.; Sahoo, B.; Sun, S.-Z.; Martin, R. Isr. J. Chem. 2020, 60, 195.
doi: 10.1002/ijch.201900072 |
|
|
(e) Wu, X.; Zhu, C. Acc. Chem. Res. 2020, 53, 1620.
doi: 10.1021/acs.accounts.0c00306 |
|
|
(f) Wang, Y.; He, Y.; Zhu, S. Acc. Chem. Res. 2022, 55, 3519.
doi: 10.1021/acs.accounts.2c00628 |
|
|
(g) Li, J.; Yu, B.; Lu, Z. Chin. J. Chem. 2021, 39, 488.
doi: 10.1002/cjoc.v39.2 |
|
| [2] |
(a) Sommer, H.; Juliá-Hernández, F.; Martin, R.; Marek, I. ACS Cent. Sci. 2018, 4, 153.
doi: 10.1021/acscentsci.8b00005 |
|
(b) Dhunganà, R. K.; Sapkota, R. R.; Niroula, D.; Giri, R. Chem. Sci. 2020, 11, 9757.
doi: 10.1039/D0SC03634J |
|
|
(c) Yin, Y.; Liu, H.; Ouyang, D. Zhang, Q.; Zhu, R. Green Synth. Catal. 2023, 4, 64.
|
|
| [3] |
(a) Han, C.; Fu, Z.; Guo, S.; Fang, X.; Lin, A.; Yao, H. ACS Catal. 2019, 9, 4196.
doi: 10.1021/acscatal.9b00688 |
|
(b) Zuo, Z.; Wang, J.; Liu, J.; Wang, Y.; Luan, X. Angew. Chem. Int. Ed. 2020, 59, 653.
doi: 10.1002/anie.v59.2 |
|
|
(c) Baumgartner, Y.; Baudoin, O. ACS Catal. 2020, 10, 10508.
doi: 10.1021/acscatal.0c02755 |
|
| [4] |
(a) He, Y.; Cai, Y.; Zhu, S. J. Am. Chem. Soc. 2017, 139, 1061.
doi: 10.1021/jacs.6b11962 pmid: 30774912 |
|
(b) Zhou, L.; Zhu, C.; Bi, P.; Feng, C. Chem. Sci. 2019, 10, 1144.
doi: 10.1039/c8sc04162h pmid: 30774912 |
|
|
(c) Lee, C.; Seo, H.; Jeon, J.; Hong, S. Nat. Commun. 2021, 12, 5657.
doi: 10.1038/s41467-021-25696-z pmid: 30774912 |
|
| [5] |
(a) Suresh, R.; Massad, I.; Marek, I. Chem. Sci. 2021, 12, 9328.
doi: 10.1039/d1sc02575a pmid: 34349902 |
|
(b) Tang, K. H. N.; Uchida, K.; Nishihara, K.; Ito, M.; Shibata, T. Org. Lett. 2022, 24, 1313.
doi: 10.1021/acs.orglett.1c04321 pmid: 34349902 |
|
|
(c) Zou, X.; Xu, S. Chin. J. Org. Chem. 2021, 41, 2610.
doi: 10.6023/cjoc202103020 pmid: 34349902 |
|
| [6] |
(a) Burkhardt, E. R.; Matos, K. Chem. Rev. 2006, 106, 2617.
doi: 10.1021/cr0406918 |
|
(b) Xu, S.; Lee, C. T.; Rao, H.; Negishia, E. I. Adv. Synth. Catal. 2011, 353, 2981.
doi: 10.1002/adsc.v353.16 |
|
|
(c) Lennox, A. J. J.; Lloyd-Jones, G. C. Chem. Soc. Rev. 2014, 43, 412.
doi: 10.1039/C3CS60197H |
|
|
(d) Neeve, E. C.; Geier, S. J.; Mkhalid, I. A. I.; Westcott, S. A.; Marder, T. B. Chem. Rev. 2016, 116, 9091.
doi: 10.1021/acs.chemrev.6b00193 |
|
|
(e) Ilies, L.; Itabashi, Y.; Shang, R.; Nakamura, E. ACS Catal. 2017, 7, 89.
doi: 10.1021/acscatal.6b02927 |
|
|
(f) Sandford, C.; Aggarwal, V. K. Chem. Commun. 2017, 53, 5481.
doi: 10.1039/C7CC01254C |
|
|
(g) Cheng, Z.; Xing, S.; Guo, J.; Cheng, B.; Hu, L.; Zhang, X.; Lu, Z. Chin. J. Chem. 2019, 37, 457.
doi: 10.1002/cjoc.v37.5 |
|
|
(h) Lu, H.; Li, B. Chin. J. Org. Chem. 2022, 42, 457. (in Chinese)
|
|
|
(陆候祥, 李必杰, 有机化学, 2022, 42, 457.)
|
|
|
(i) Lu, H.; Li, B. Chin. J. Org. Chem. 2022, 42, 3167. (in Chinese)
doi: 10.6023/cjoc202207040 |
|
|
(陆候祥, 李必杰, 有机化学, 2022, 42, 3167.)
doi: 10.6023/cjoc202207040 |
|
| [7] |
(a) Obligacion, J. V.; Chirik, P. J. J. Am. Chem. Soc. 2013, 135, 19107.
doi: 10.1021/ja4108148 pmid: 30258070 |
|
(b) Ruddy, A. J.; Sydora, O. L.; Small, B. L.; Stradiotto, M.; Turculet, L. Chem.-Eur. J. 2014, 20, 13918.
doi: 10.1002/chem.201403945 pmid: 30258070 |
|
|
(c) Scheuermann, M. L.; Johnson, E. J.; Chirik, P. J. Org. Lett. 2015, 17, 2716.
doi: 10.1021/acs.orglett.5b01135 pmid: 30258070 |
|
|
(d) Palmer, W. N.; Obligacion, J. V.; Pappas, I.; Chirik, P. J. J. Am. Chem. Soc. 2016, 138, 766.
doi: 10.1021/jacs.5b12249 pmid: 30258070 |
|
|
(e) Chen, X.; Cheng, Z.; Guo, J.; Lu, Z. Nat. Commun. 2018, 9, 3939.
doi: 10.1038/s41467-018-06240-y pmid: 30258070 |
|
|
(f) Hu, M.; Ge, S. Nat. Commun. 2020, 11, 765.
doi: 10.1038/s41467-020-14543-2 pmid: 30258070 |
|
| [8] |
Lei, Y.; Huang, J.; Zhao, W. Org. Lett. 2021, 23, 7797.
doi: 10.1021/acs.orglett.1c02826 |
| [9] |
(a) Michael, J. P. Nat. Prod. Rep. 2002, 19, 719.
pmid: 27689804 |
|
(b) Saibabu Kotti, S. R.; Timmons, C.; Li, G. Chem. Biol. Drug. Des. 2006, 67, 101.
pmid: 27689804 |
|
|
(c) Plechkova, N. V.; Seddon, K. R. Chem. Soc. Rev. 2008, 37, 123.
doi: 10.1039/b006677j pmid: 27689804 |
|
|
(d) McGrath, N. A.; Brichacek, M.; Njardarson, J. T. J. Chem. Educ. 2010, 87, 1348.
doi: 10.1021/ed1003806 pmid: 27689804 |
|
|
(e) Ruiz-Castillo, P.; Buchwald, S. L. Chem. Rev. 2016, 116, 12564.
pmid: 27689804 |
|
| [10] |
Bélanger, G.; Doré, M.; Ménard, F.; Darsigny, V. J. Org. Chem. 2006, 71, 7481.
doi: 10.1021/jo0611061 |
| [11] |
He, Y.; Song, H.; Chen, J.; Zhu, S. Nat. Commun. 2021, 12, 638.
doi: 10.1038/s41467-020-20888-5 |
| [12] |
Hosokawa, S.; Teramoto, K.; Motoyama, Y. ChemistrySelect 2016, 1, 2594.
doi: 10.1002/slct.201600552 |
| [13] |
Richa; Kumar, R.; Zhang, X.; Su, W. Org. Chem. Front. 2020, 7, 2965.
doi: 10.1039/D0QO00911C |
| [14] |
Fisher, G. B.; Lee, L.; Klettke, F. W. Synth. Commun. 1994, 24, 1541.
doi: 10.1080/00397919408010154 |
| [15] |
Fukumoto, Y.; Asai, H.; Shimizu, M.; Chatani, N. J. Am. Chem. Soc. 2007, 129, 13792.
pmid: 17949090 |
| [16] |
Alcaide, B.; Almendros, P.; Alonso, J. M.; Aly, M. F. Org. Lett. 2001, 3, 3781.
pmid: 11700137 |
| [17] |
Crotti, P.; Favero, L.; Macchia, F.; Pineschi, M. Tetrahedron Lett. 1994, 35, 7089.
doi: 10.1016/0040-4039(94)88233-9 |
| [18] |
Fu, R.; Liu, Y.; Wu, T.; Zhang, X.; Zhu, Y.; Luo, J.; Zhang, Z.; Jiang, Y. Chem. Commun. 2022, 58, 3525.
doi: 10.1039/D2CC00169A |
| [19] |
Ueno, S.; Usui, K.; Kuwano, R. Synlett 2011, 1303.
|
| [20] |
Pan, Y.; You, Y.; He, D.; Chen, F.; Chang, X.; Jin, M. Y.; Xing, X. Org. Lett. 2020, 22, 7278.
doi: 10.1021/acs.orglett.0c02614 |
| [21] |
Bhadra, S.; Yamamoto, H. Angew. Chem. Int. Ed. 2016, 55, 13043.
doi: 10.1002/anie.201606354 |
| [22] |
Nakamura, Y.; Ohta, T.; Oe, Y. Chem. Commun. 2015, 51, 7459.
doi: 10.1039/C5CC01584G |
| [23] |
Das, K.; Sarkar, K.; Maji, B. ACS Catal. 2021, 11, 7060.
doi: 10.1021/acscatal.1c01199 |
| [24] |
Wei, H. X.; Lu, D.; Sun, V.; Zhang, J.; Gu, Y.; Osenkowski, P.; Ye, W.; Selkoe, D. J.; Wolfe, M. S.; Augelli-Szafran, C. E. Bioorg. Med. Chem. Lett. 2016, 26, 2133.
doi: 10.1016/j.bmcl.2016.03.042 |
| [25] |
Stütz, A.; Georgopoulos, A.; Granitzer, W.; Petranyi, G.; Berney, D. J. Med. Chem. 1986, 29, 112.
pmid: 3510297 |
| [26] |
Singh, B.; Chetia, D. Kumawat, M. K. Pharm. Chem. J. 2021, 55, 724.
doi: 10.1007/s11094-021-02484-z |
| [27] |
Luo, L.; Song, Q.; Li, Y.; Cao, Z.; Qiang, X.; Tan, Z.; Deng, Y. Bioorg. Med. Chem. 2020, 28, 115400.
doi: 10.1016/j.bmc.2020.115400 |
| [28] |
Bai, X. Y.; Zhao, W.; Sun, X.; Li, B. J. J. Am. Chem. Soc. 2019, 141, 19870.
doi: 10.1021/jacs.9b10578 |
| [29] |
Sun, S. Z.; Martin, R. Angew. Chem., Int. Ed. 2018, 57, 3622.
doi: 10.1002/anie.201712428 |
| [30] |
Jarava-Barrera, C.; Parra, A.; López, A.; Cruz-Acosta, F.; Collado-Sanz, D.; Cárdenas, D. J.; Tortosa, M. ACS Catal. 2016, 6, 442.
pmid: 27088045 |
| [31] |
Mlynarski, S. N.; Karns, A. S.; Morken, J. P. J. Am. Chem. Soc. 2012, 134, 16449.
doi: 10.1021/ja305448w pmid: 23002712 |
| [32] |
Yang, C.; Gao, Y.; Bai, S.; Jiang, C.; Qi, X. J. Am. Chem. Soc. 2020, 142, 11506.
doi: 10.1021/jacs.0c03821 |
| [1] | Wenfeng Bei, Jian Pan, Dongmei Ran, Yilin Liu, Zhen Yang, Ruokun Feng. Cobalt-Catalyzed [4+2] Annulation of Indole Carboxamide with Diynes and Monoacetylene: Direct Access to γ-Carbolinones [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3226-3238. |
| [2] | Shuang Liu, Lianghua Zou, Xiaoming Wang. Advance of Dehydrogenation and Transfer Hydrogenation of Ammonia-Borane Catalyzed by Homogeneous Cobalt Complexes [J]. Chinese Journal of Organic Chemistry, 2023, 43(5): 1713-1725. |
| [3] | Shiquan Gao, Chuangjun Liu, Junfeng Yang, Junliang Zhang. Cobalt-Catalyzed Electrochemical Reductive Coupling of Alkynes and Alkenes [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1559-1565. |
| [4] | Peng Zhou, Weiming Zhu, Jiantao Zhang, Duoduo Xiao, Xiangfeng Guo, Weibing Liu. Cobalt-Catalyzed Oxyalkylation Reaction of Styrenes: Rapid Access to α-Alkyl Substituted Acetophenone Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3939-3944. |
| [5] | Zehui Li, Haoyu Zou, Lincai Li, Yiling Zhao, Hongping Zhu. Synthesis and Propylene Oxide Carbonylation Hydroesterification Catalytic Property of N,O-Ligand Coordination Cobalt Compounds [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3907-3915. |
| [6] | Chaoyu Wang, Shuda Dong, Tianyang Zhu, Yuqin Liu, Zihan Wu, Ruokun Feng. Cobalt-Catalyzed Decarbonylative C(8)-Acyloxylation of 1-Naphthalamine Derivatives with α-Oxocarboxylic Acids [J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1799-1810. |
| [7] | Lianrong Fu, Yan-Bing Wang, Hui Jiang, Xin-Qi Hao, Mao-Ping Song. Applications of Cobalt Complexes in Olefin Polymerization [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3530-3548. |
| [8] | Yinyin Wang, Xiaowan Lin, Piao Zhang, Meihua Shen, Huadong Xu, Defeng Xu. Design and Synthesis of Pyridine and 1,3,5-Triazine PNP Pincer Ligands and Their Application in Cobalt Catalyzed Semihydrogenation of Terminal Alkynes [J]. Chinese Journal of Organic Chemistry, 2021, 41(8): 3312-3320. |
| [9] | Yinjun Huang, Jinshan Li, Shen Li, Junan Ma. Cobalt-Catalyzed Aerobic Oxidative Dearomatization of 2-Aryl Indoles and in situ [3+2] Annulation with Enamides [J]. Chinese Journal of Organic Chemistry, 2021, 41(10): 4028-4038. |
| [10] | Ya-Lan Feng, Bing-Feng Shi. Recent Advances in Base Metal (Copper, Cobalt and Nickel)-Catalyzed Directed C—H Amination [J]. Chinese Journal of Organic Chemistry, 2021, 41(10): 3753-3770. |
| [11] | Dai Zinan, Yu Zehao, Bai Ying, Li Jiayun, Peng Jiajian. Progress in Catalysis of Hydrosilylation by Cobalt Complexes [J]. Chinese Journal of Organic Chemistry, 2020, 40(5): 1177-1187. |
| [12] | Sun Yue, Guan Rui, Liu Zhaohong, Wang Yeming. Recent Advances in Hydroboration of Alkenes Catalyzed by Fe, Co and Ni [J]. Chinese Journal of Organic Chemistry, 2020, 40(4): 899-912. |
| [13] | Zhang Mengfan, Li Ruipeng, Yang Zhen, Feng Ruokun. Cobalt-Catalyzed Bidentate-Assisted Regioselective C—H Alkoxylation of 1-Naphthylamide with Alcohols [J]. Chinese Journal of Organic Chemistry, 2020, 40(3): 714-723. |
| [14] | Cheng Biao, Lu Peng, Zhao Jiajin, Lu Zhan. Cobalt-Catalyzed Dehydrogenative Silylation of Vinylarenes [J]. Chin. J. Org. Chem., 2019, 39(6): 1704-1710. |
| [15] | Sun Yiming, Ding Qifeng, Yu Yang, He Yide, Huang Fei. Progress in Co-Catalyzed C-H Amination [J]. Chinese Journal of Organic Chemistry, 2019, 39(12): 3363-3374. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||