Chinese Journal of Organic Chemistry ›› 2023, Vol. 43 ›› Issue (9): 3146-3166.DOI: 10.6023/cjoc202308001 Previous Articles Next Articles
收稿日期:2023-08-01
修回日期:2023-09-12
发布日期:2023-09-21
基金资助:Received:2023-08-01
Revised:2023-09-12
Published:2023-09-21
Contact:
E-mail: Supported by:Share
Wenfang Wang. Recent Progress in Transition-Metal-Catalyzed Asymmetric C—H Borylation[J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3146-3166.
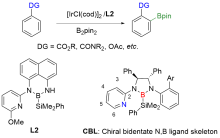
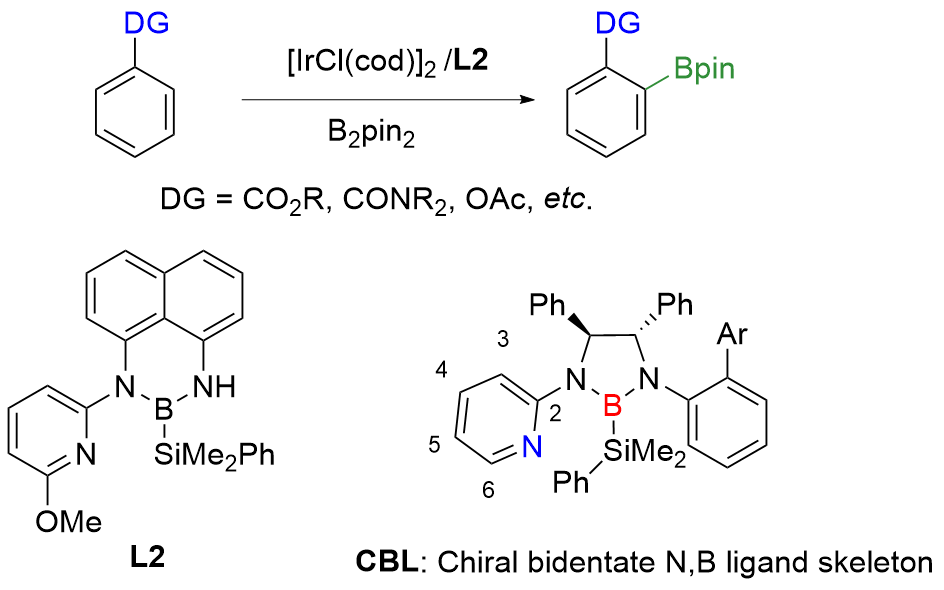
| Entry | R1, R2, R3 | Conv./% | ee(6')/% | ee(5')/% | sa |
|---|---|---|---|---|---|
| 1 | CF3, CF3, H | 30 | 94 | 41 | 68 |
| 2 | CF3, CF3, 3-Me | 30 | 90 | 40 | 25 |
| 3 | OMe, OMe, H | 33 | 85 | 42 | 19 |
| 4 | OMe, CF3, 3-Cl | 50 | 88 | 88 | 45 |
| 5 | CF3, CF3, 4-Cl | 54 | 72 | 62 | 9 |
| Entry | R1, R2, R3 | Conv./% | ee(6')/% | ee(5')/% | sa |
|---|---|---|---|---|---|
| 1 | CF3, CF3, H | 30 | 94 | 41 | 68 |
| 2 | CF3, CF3, 3-Me | 30 | 90 | 40 | 25 |
| 3 | OMe, OMe, H | 33 | 85 | 42 | 19 |
| 4 | OMe, CF3, 3-Cl | 50 | 88 | 88 | 45 |
| 5 | CF3, CF3, 4-Cl | 54 | 72 | 62 | 9 |
| Entry | B2pin2/equiv. | 10a∶I∶II∶IIIa | Yielda/% | eeb/% |
|---|---|---|---|---|
| 1 | 1.00 | 87.6∶0.0∶9.9∶2.5 | 91 (83)c | 92 |
| 2 | 0.50 | 98.8∶0.0∶1.2∶0.0 | 65 | 87 |
| 3 | 0.75 | 96.2∶0.0∶3.1∶0.7 | 88 | 88 |
| 4 | 1.30 | 57.7∶0.0∶38.8∶3.5 | 53 (52)c | 96 |
| Entry | B2pin2/equiv. | 10a∶I∶II∶IIIa | Yielda/% | eeb/% |
|---|---|---|---|---|
| 1 | 1.00 | 87.6∶0.0∶9.9∶2.5 | 91 (83)c | 92 |
| 2 | 0.50 | 98.8∶0.0∶1.2∶0.0 | 65 | 87 |
| 3 | 0.75 | 96.2∶0.0∶3.1∶0.7 | 88 | 88 |
| 4 | 1.30 | 57.7∶0.0∶38.8∶3.5 | 53 (52)c | 96 |
| [1] |
(a) Sun H.-Y.; Hall D. G. In Synthesis and Application of Organoboron Compounds, Eds.: Fernandez, E.; Whiting, A., Springer International, Cham, 2015, p. 221.
|
|
(b) Leonori D.; Aggarwal V. K. In Synthesis and Application of Organoboron Compounds, Eds.: Fernandez, E.; Whiting, A., Springer International, Cham, 2015, p. 271.
|
|
|
(c) Jakle F. In Synthesis and Application of Organoboron Compounds, Eds.: Fernandez, E.; Whiting, A., Springer International, Cham, 2015, p. 297.
|
|
|
(d) Wang M.; Shi Z. Chem. Rev. 2020, 120, 7348.
doi: 10.1021/acs.chemrev.9b00384 |
|
|
(e) Hall D. G. Boronic Acids: Preparation and Applications in Organic Synthesis Medicine and Materials, Ed.: Hall, D. G., Wiley-VCH, Weinheim, 2011. p. 28.
|
|
|
(f) Cui P.-F.; Gao Y.; Guo S.-T.; Jin G.-X. Chin. J. Chem. 2021, 39, 281.
doi: 10.1002/cjoc.v39.2 |
|
|
(g) Wang Q.-T.; Meng W.; Feng X.-Q.; Du H.-F. Chin. J. Chem. 2021, 39, 918.
doi: 10.1002/cjoc.v39.4 |
|
| [2] |
Xie T.; Chen L.-L.; Shen Z.-L; Xu S.-M. Angew. Chem., Int. Ed. 2023, 62, e202300199.
|
| [3] |
Su B.; Zhou T.-G.; Xu P.-L.; Shi Z.-J.; Hartwig J. F. Angew. Chem., Int. Ed. 2017, 56, 7205.
|
| [4] |
Shi Y.-J.; Gao Q.; Xu S.-M. J. Am. Chem. Soc. 2019, 141, 10599.
doi: 10.1021/jacs.9b04549 |
| [5] |
Dick L. R.; Fleming P. E. Drug Discovery Today 2010, 15, 243.
doi: 10.1016/j.drudis.2010.01.008 |
| [6] |
Adams J.; Kauffman M. Cancer Invest. 2004, 22, 304.
doi: 10.1081/CNV-120030218 |
| [7] |
Gentile M.; Offidani M.; Vigna E.; Corvatta L.; Recchia A. G.; Morabito L.; Morabito F.; Gentili S. Expert Opin. Invest. Drugs 2015, 24, 1287.
doi: 10.1517/13543784.2015.1065250 |
| [8] |
Gallerani E.; Zucchetti M.; Brunelli D.; Marangon E.; Noberasco C.; Hess D.; Delmonte A.; Martinelli G.; Bohm S.; Driessen C.; De Braud F.; Marsoni S.; Cereda R.; Sala F.; D'Incalci M.; Sessa C. Eur. J. Cancer 2013, 49, 290.
doi: 10.1016/j.ejca.2012.09.009 pmid: 23058787 |
| [9] |
Roemmele R. C.; Christie M. A. Org. Process Res. Dev. 2013, 17, 422.
doi: 10.1021/op400010u |
| [10] |
(a) Jin S.; Zhu C.; Li M.; Wang B. Bioorg. Med. Chem. Lett. 2009, 19, 1596.
doi: 10.1016/j.bmcl.2009.02.011 |
|
(b) Jin S.; Zhu C.; Cheng Y.; Li M.; Wang B. Bioorg. Med. Chem. 2010, 18, 1449.
doi: 10.1016/j.bmc.2010.01.017 |
|
| [11] |
Andres P.; Ballano G.; Calaza M. I.; Cativiela C. Chem. Soc. Rev. 2016, 45, 2291.
doi: 10.1039/C5CS00886G |
| [12] |
(a) Brown H. C.; Cole T. E. Organometallics 1983, 2, 1316.
doi: 10.1021/om50004a009 pmid: 17849483 |
|
(b) Burgess K.; Van der Donk W. A.; Westcott S. A.; Marder T. B.; Baker R. T.; Calabrese J. C. J. Am. Chem. Soc. 1992, 114, 9350.
doi: 10.1021/ja00050a015 pmid: 17849483 |
|
|
(c) Crudden C. M.; Edwards D. Eur. J. Org. Chem. 2003, 2003, 4695.
doi: 10.1002/ejoc.v2003:24 pmid: 17849483 |
|
|
(d) Burgess K.; Ohlmeyer M. J. Chem. Rev. 1991, 91, 1179.
doi: 10.1021/cr00006a003 pmid: 17849483 |
|
|
(e) Matteson D. S.; Sadhu K. M.; Lienhard G. E. J. Am. Chem. Soc. 1981, 103, 5241.
doi: 10.1021/ja00407a051 pmid: 17849483 |
|
|
(f) Matteson D. S. Chem. Rev. 1989, 89, 1535.
doi: 10.1021/cr00097a009 pmid: 17849483 |
|
|
(g) Mantri P.; Duffy D. E.; Kettner C. A. J. Org. Chem. 1996, 61, 5690.
doi: 10.1021/jo960628l pmid: 17849483 |
|
|
(h) Carmes L.; Carreaux F.; Carboni B.; Mortier J. Tetrahedron Lett. 1998, 39, 555.
doi: 10.1016/S0040-4039(97)10682-7 pmid: 17849483 |
|
|
(i) Lebarbier C.; Carreaux F.; Carboni B.; Boucher J. L. Bioorg. Med. Chem. Lett. 1998, 8, 2573.
pmid: 17849483 |
|
|
(j) Singh R. P.; Matteson D. S. J. Org. Chem. 2000, 65, 6650.
pmid: 17849483 |
|
|
(k) Jagannathan S.; Forsyth T. P.; Kettner C. A. J. Org. Chem. 2001, 66, 6375.
pmid: 17849483 |
|
|
(l) Matteson D. S. Med. Res. Rev. 2008, 28, 233.
pmid: 17849483 |
|
|
(m) Matteson D. S.; Maliakal D.; Fabry-Asztalos L. J. Organomet. Chem. 2008, 693, 2258.
doi: 10.1016/j.jorganchem.2008.03.031 pmid: 17849483 |
|
|
(n) Batsanov A. S.; Grosjean C.; Schutz T.; Whiting A. J. Org. Chem. 2007, 72, 6276.
pmid: 17849483 |
|
| [13] |
(a) Wang Z.; Bachman S.; Dudnik A. S.; Fu G. C. Angew. Chem., Int. Ed. 2018, 57, 14529.
pmid: 19653692 |
|
(b) Cheng Q. Q.; Zhu S. F.; Zhang Y. Z.; Xie X. L.; Zhou Q. L. J. Am. Chem. Soc. 2013, 135, 14094.
doi: 10.1021/ja408306a pmid: 19653692 |
|
|
(c) Joannou M. V.; Moyer B. S.; Meek S. J. J. Am. Chem. Soc. 2015, 137, 6176.
doi: 10.1021/jacs.5b03477 pmid: 19653692 |
|
|
(d) Chen D.; Zhang X.; Qi W. Y.; Xu B.; Xu M.-H. J. Am. Chem. Soc. 2015, 137, 5268.
doi: 10.1021/jacs.5b00892 pmid: 19653692 |
|
|
(e) Zhan M.; Li R.-Z.; Mou Z. D.; Cao C.-G.; Liu J.; Chen Y.-W.; Niu D.-W. ACS Catal. 2016, 6, 3381.
doi: 10.1021/acscatal.6b00719 pmid: 19653692 |
|
|
(f) Potter B.; Szymaniak A. A.; Edelstein E. K.; Morken J. P. J. Am. Chem. Soc. 2014, 136, 17918.
doi: 10.1021/ja510266x pmid: 19653692 |
|
|
(g) Sun C.; Potter B.; Morken J. P. J. Am. Chem. Soc. 2014, 136, 6534.
doi: 10.1021/ja500029w pmid: 19653692 |
|
|
(h) Smilovic I. G.; Casas-Arce E.; Roseblade S. J.; Nettekoven U.; Zanotti-Gerosa A.; Kovacevic M.; Casar Z. Angew. Chem., Int. Ed. 2012, 51, 1014.
pmid: 19653692 |
|
|
(i) Chen I.-H.; Yin L.; Itano W.; Kanai M.; Shibasaki M. J. Am. Chem. Soc. 2009, 131, 11664.
doi: 10.1021/ja9045839 pmid: 19653692 |
|
|
(j) Sim H.-S.; Feng X.; Yun J. Chem.-Eur. J. 2009, 15, 1939.
doi: 10.1002/chem.v15:8 pmid: 19653692 |
|
|
(k) Kitanosono T.; Xu P.; Isshiki S.; Zhu L.; Kobayashi S. Chem. Commun. 2014, 50, 9336.
doi: 10.1039/C4CC04062G pmid: 19653692 |
|
|
(l) Lee J. E.; Yun J. Angew. Chem., Int. Ed. 2008, 47, 145.
pmid: 19653692 |
|
|
(m) Kitanosono T.; Kobayashi S. Asian J. Org. Chem. 2013, 2, 961.
doi: 10.1002/ajoc.v2.11 pmid: 19653692 |
|
|
(n) O'Brien, J. M.; Lee, K.-S.; Hoveyda, A. H. J. Am. Chem. Soc. 2010, 132, 10630.
doi: 10.1021/ja104777u pmid: 19653692 |
|
| [14] |
(a) Woźniak Ł.; Tan J. F.; Nguyen Q. H.; Madron du Vigné A.; Smal V.; Cao Y. X.; Cramer N. Chem. Rev. 2020, 120, 10516.
doi: 10.1021/acs.chemrev.0c00559 pmid: 32897713 |
|
(b) Zhao M.; Song P.-D.; Jiao J.; Li P.-F. Chin. J. Chem. 2020, 38, 665.
doi: 10.1002/cjoc.v38.6 pmid: 32897713 |
|
|
(c) Wang X.-Y.; Zhang P.-F.; Ye M.-C. Chin. J. Chem. 2020, 38, 1762.
doi: 10.1002/cjoc.v38.12 pmid: 32897713 |
|
|
(d) Wang W.-F.; Sun W. Chin. J. Org. Chem. 2023, 43, 1605. (in Chinese)
doi: 10.6023/cjoc202300023 pmid: 32897713 |
|
|
(王文芳, 孙伟, 有机化学, 2023, 43, 1605.)
doi: 10.6023/cjoc202300023 pmid: 32897713 |
|
| [15] |
(a) Su B.; Hartwig J. F. Angew. Chem., Int. Ed. 2022, 61, e202113343.
doi: 10.1002/anie.v61.9 |
|
(b) Tamura H.; Yamazaki H.; Sato H.; Sakaki S. J. Am. Chem. Soc. 2003, 125, 16114.
doi: 10.1021/ja0302937 |
|
|
(c) Boller T. M.; Murphy J. M.; Hapke M.; Ishiyama T.; Miyaura N.; Hartwig J. F. J. Am. Chem. Soc. 2005, 127, 14263.
doi: 10.1021/ja053433g |
|
| [16] |
Park D.; Baek D.; Lee C.-W.; Ryu H.; Park S.; Han W.; Hong S. Tetrahedron 2021, 79, 131811.
doi: 10.1016/j.tet.2020.131811 |
| [17] |
Baek D.; Ryu H.; Ryu J. Y.; Lee J.; Stoltz B. M.; Hong S. Chem. Sci. 2020, 11, 4602.
doi: 10.1039/D0SC00412J |
| [18] |
Wang G.-H.; Liu L.; Wang H.; Ding Y. S.; Zhou J.; Mao S.; Li P.-F. J. Am. Chem. Soc. 2017, 139, 91.
doi: 10.1021/jacs.6b11867 |
| [19] |
Zou X.-L.; Zhao H.-N.; Li Y.-W.; Gao Q.; Ke Z.-F.; Xu S.-M. J. Am. Chem. Soc. 2019, 141, 5334.
doi: 10.1021/jacs.8b13756 |
| [20] |
Lemouzy S.; Giordano L.; Hérault D.; Buono G. Eur. J. Org. Chem. 2020, 2020, 3351.
doi: 10.1002/ejoc.v2020.23 |
| [21] |
Song S.-Y.; Li Y.-W.; Ke Z.-F.; Xu S.-M. ACS Catal. 2021, 11, 13445.
doi: 10.1021/acscatal.1c03888 |
| [22] |
Jing K.; Chen L.-L.; Zhang P.; Xu S.-M. Chin. J. Chem. 2023, 41, 2119.
doi: 10.1002/cjoc.v41.17 |
| [23] |
Song S.-Y.; Zhou X.-Y.; Ke Z.-F.; Xu S.-M. Angew. Chem., Int. Ed. 2023, 62, e202217130.
|
| [24] |
(a) Kim B.; Chinn A. J.; Fandrick D. R.; Senanayake C. H.; Singer R. A.; Miller S. J. J. Am. Chem. Soc. 2016, 138, 7939.
doi: 10.1021/jacs.6b03444 |
|
(b) Metrano A. J.; Miller S. J. Acc. Chem. Res. 2019, 52, 199.
doi: 10.1021/acs.accounts.8b00473 |
|
| [25] |
Genov G. R.; Douthwaite J. L.; Lahdenperä A. S. K.; Gibson D. C.; Phipps R. J. Science 2020, 367, 1246.
doi: 10.1126/science.aba1120 |
| [26] |
Song P.-D.; Hu L.-L.; Yu T.; Jiao J.; He Y.-Q.; Xu L.; Li P.-F. ACS Catal. 2021, 11, 7339.
doi: 10.1021/acscatal.1c01671 |
| [27] |
(a) Fu G. C. Acc. Chem. Res. 2006, 39, 853.
doi: 10.1021/ar068115g |
|
(b) Rios R.; Liang J.; Lo M. M. C.; Fu G. C. Chem. Commun. 2000, 377.
|
|
| [28] |
Lötscher D.; Rupprecht S.; Stoeckli-Evans H.; von Zelewsky A. Tetrahedron: Asymmetry 2000, 11, 4341.
doi: 10.1016/S0957-4166(00)00401-8 |
| [29] |
(a) Fu G. C. Acc. Chem. Res. 2004, 37, 542.
doi: 10.1021/ar030051b |
|
(b) Fu G. C. Acc. Chem. Res. 2000, 33, 412.
doi: 10.1021/ar990077w |
|
|
(c) Gomez Arrayas R.; Adrio J.; Carretero J. C. Angew. Chem., Int. Ed. 2006, 45, 7674.
doi: 10.1002/(ISSN)1521-3773 |
|
|
(d) Dai L.-X.; Tu T.; You S.-L.; Deng W.-P.; Hou X.-L. Acc. Chem. Res. 2003, 36, 659.
doi: 10.1021/ar020153m |
|
| [30] |
Zou X.-L.; Li Y.-W.; Ke Z.-F.; Xu S.-M. ACS Catal. 2022, 12, 1830.
doi: 10.1021/acscatal.1c05299 |
| [31] |
(a) Newton C. G.; Wang S. G.; Oliveira C. C.; Cramer N. Chem. Rev. 2017, 117, 8908.
doi: 10.1021/acs.chemrev.6b00692 |
|
(b) Saint-Denis T. G.; Zhu R.-Y.; Chen G.; Wu Q.-F.; Yu J.-Q. Science 2018, 359, eaao4798.
doi: 10.1126/science.aao4798 |
|
| [32] |
(a) He J.; Jiang H.; Takise R.; Zhu R.-Y.; Chen G.; Dai H.-X.; Dhar T. G. M.; Shi J.; Zhang H.; Cheng P. T.; Yu J.-Q. Angew. Chem., Int. Ed. 2016, 55, 785.
|
|
(b) Zhang L.-S.; Chen G.-H.; Wang X.; Guo Q.-Y.; Zhang X.-S.; Pan F.; Chen K.; Shi Z.-J. Angew. Chem., Int. Ed. 2014, 53, 3899.
doi: 10.1002/anie.201310000 |
|
| [33] |
He J.; Shao Q.; Wu Q.-F.; Yu J.-Q., J. J. Am. Chem. Soc. 2017, 139, 3344.
doi: 10.1021/jacs.6b13389 |
| [34] |
Chen X.; Chen L.-L.; Zhao H.-L.; Gao Q.; Shen Z.-L.; Xu S.-M. Chin. J. Chem. 2020, 38, 1533.
doi: 10.1002/cjoc.v38.12 |
| [35] |
Ebner C.; Carreira E. M. Chem. Rev. 2017, 117, 11651.
doi: 10.1021/acs.chemrev.6b00798 |
| [36] |
(a) Gagnon A.; Duplessis M.; Fader L. Org. Prep. Proced. Int. 2010, 42, 1.
doi: 10.1080/00304940903507788 |
|
(b) Talele T. T. J. Med. Chem. 2016, 59, 8712.
doi: 10.1021/acs.jmedchem.6b00472 |
|
| [37] |
Wu W.-Q.; Lin Z.-M.; Jiang H.-F. Org. Biomol. Chem. 2018, 16, 7315.
doi: 10.1039/C8OB01187G |
| [38] |
Shi Y.-J.; Yang Y.-H.; Xu S.-M. Angew. Chem., Int. Ed. 2022, 61, e202201463.
|
| [39] |
Gao Q.; Xu S.-M. Angew. Chem., Int. Ed. 2023, 62, e202218025.
doi: 10.1002/anie.v62.8 |
| [40] |
Chen L.-L.; Yang Y.-H.; Liu L.-H.; Gao Q.; Xu S.-M. J. Am. Chem. Soc. 2020, 142, 12062.
doi: 10.1021/jacs.0c06756 |
| [41] |
Giri R.; Shi B.-F.; Engle K. M.; Maugel N.; Yu J.-Q. Chem. Soc. Rev. 2009, 38, 3242.
doi: 10.1039/b816707a |
| [42] |
(a) Kang E.; Kim H. T.; Joo J. M. Org. Biomol. Chem. 2020, 18, 6192.
doi: 10.1039/D0OB01265C |
|
(b) Lee W.-G. C.; Shen Y.; Gutierrez D. A.; Li J. J. Org. Lett. 2016, 18, 2660.
doi: 10.1021/acs.orglett.6b01105 |
|
|
(c) Shen Y.; Lee W.-G. C.; Gutierrez D. A.; Li J. J. J. Org. Chem. 2017, 82, 11620.
doi: 10.1021/acs.joc.7b01883 |
|
|
(d) Yuan C.; Tu G.; Zhao Y. Org. Lett. 2017, 19, 356.
|
|
|
(e) Kim H.; Thombal R. S.; Khanal H. D.; Lee Y. R. Chem. Commun. 2019, 55, 13402.
doi: 10.1039/C9CC06758B |
|
| [43] |
Kashima C.; Hibi S.; Maruyama T.; Harada K.; Omote Y. J. Heterocycl. Chem. 1987, 24, 637.
doi: 10.1002/jhet.v24:3 |
| [44] |
Du R.-R.; Liu L.-H.; Xu S.-M. Angew. Chem., Int. Ed. 2021, 60, 5843.
doi: 10.1002/anie.v60.11 |
| [45] |
Yang Y.-H.; Chen L.-L.; Xu S.-M. Angew. Chem., Int. Ed. 2021, 60, 3524.
doi: 10.1002/anie.v60.7 |
| [46] |
Chen X.; Cheng Z.-Y.; Guo J.; Lu Z. Nat. Commun. 2018, 9, 3939.
doi: 10.1038/s41467-018-06240-y pmid: 30258070 |
| [47] |
Zhang M.-H.; Ye Z.-Y.; Zhao W.-X. Angew. Chem., Int. Ed. 2023, 62, e202306248.
doi: 10.1002/anie.v62.31 |
| [1] | Xuqin Zhong, Zhen Liu. Conformational Screening of the Catalyst System Containing Transition Metal and Flexible Ligand [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 734-741. |
| [2] | Li Dai, Di Xu, Yifei Mao, Jiaqi Zhu, Mengjiao Yang. Structures and Synthetic Strategies of Chiral Oxazolinyl Ferrocene Derivatives [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2364-2375. |
| [3] | Hu Zhixiong, Sun Dongdong, Han Xie, Liu Simin. Molecular Recognition of Cucurbit[10]uril toward Planar d8 and d10 Transition Metal Complexes [J]. Chinese Journal of Organic Chemistry, 2020, 40(5): 1361-1366. |
| [4] | Xu Shuanghua, Chen Jun, Chen Jiarong, Xiao Wenjing. Recent Progress in Applications of Cinchona Alkaloids and Their Derivatives in Asymmetric Catalysis [J]. Chinese Journal of Organic Chemistry, 2020, 40(11): 3493-3516. |
| [5] | Wang Mincan. Enantioselective Analysis: Logic of Chiral Ligand Design for Asymmetric Addition of Diethylzinc to Benzaldehyde [J]. Chin. J. Org. Chem., 2018, 38(1): 162-170. |
| [6] | Qin Xiaofei, Liu Xiaoyan, Guo Caihong, Wu Haishun. Reaction Mechanisms of Carbonyl Compounds Hydrosilylation Catalyzed by Group VIII Transition Metal Complexes [J]. Chin. J. Org. Chem., 2016, 36(1): 60-71. |
| [7] | Zhu Xueyou, Li Xiaohong, Han Fengjiao, Zhang Shichen, Zhao Haiying, Bian Zhanxi. Burning-Rate Catalytic Properties of Ferrocenyl β-Diketones and Their Cu(II), Ni(II) Complexes [J]. Chin. J. Org. Chem., 2015, 35(4): 922-926. |
| [8] | Fu Ying, Hou Bo, Zhao Xingling, Du Zhengyin, Hu Yulai. Progress on the Asymmetric Silylcyanation of Aldehydes and Ketones [J]. Chin. J. Org. Chem., 2015, 35(12): 2507-2521. |
| [9] | Zheng Longsheng, Song Tao, Xu Liwen. 1,1’-Binaphthalene-2-α-arylmethanol-2’-ols (Ar-BINMOLs) with Axial and sp3-Central Chirality:A Novel Chiral Ligands for Catalytic Asymmetric Transformations [J]. Chin. J. Org. Chem., 2014, 34(7): 1255-1267. |
| [10] | Song Shasha, Zhou Hongyong, Li Xiaona, Wang Lihua, Li Yunqing, Wang Jiaxi. Advance of Glucosamine and Their Derivatives as Chiral Ligands in Asymmetric Syntheses [J]. Chin. J. Org. Chem., 2014, 34(4): 706-716. |
| [11] | Xue Feng, Li Changgong, Chen Jie, Wan Boshun. Recent Progress of Sulfoxide and N-Sulfinyl Ligands in Asymmetric Catalysis [J]. Chin. J. Org. Chem., 2014, 34(2): 267-277. |
| [12] | Wang Dong, Hou Chuanjin, Chen Lifeng, Liu Xiaoning, An Qingda, Hu Xiangping. Progress on the Catalytic Asymmetric Hydrogenation of Imines [J]. Chin. J. Org. Chem., 2013, 33(07): 1355-1368. |
| [13] | Fang Huayi, Ling Zhen, Fu Xuefeng. N—H Bond Activation of Ammonia by Transition Metal and Main Group Element Complexes [J]. Chin. J. Org. Chem., 2013, 33(04): 738-748. |
| [14] | Cui Penglei, Liu Huimin, Guo Xiumin, Zhang Dongnuan, Wang Yanen, Wang Chun. Application of Sugar-Based Phosphorus Ligands in Asymmetric Hydrogenation [J]. Chin. J. Org. Chem., 2013, 33(03): 436-443. |
| [15] | YANG Wang-Guia, WU Shuaia, SHI Min*,a,b. Progress of Organozinc Reagents in Asymmetric Addition Reactions Catalyzed by Sulfur-Containing Chiral Ligands [J]. Chin. J. Org. Chem., 2007, 27(02): 197-208. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
