Chinese Journal of Organic Chemistry ›› 2024, Vol. 44 ›› Issue (3): 871-891.DOI: 10.6023/cjoc202310033 Previous Articles Next Articles
Special Issue: 光电催化综述合集
REVIEWS
收稿日期:2023-10-31
修回日期:2023-12-13
发布日期:2024-04-02
作者简介:共同第一作者
基金资助:
Zile Zhu, Pengfei Li, Youai Qiu( )
)
Received:2023-10-31
Revised:2023-12-13
Published:2024-04-02
Contact:
*E-mail: qiuyouai@nankai.edu.cn
About author:The authors contributed equally to this work.
Supported by:Share
Zile Zhu, Pengfei Li, Youai Qiu. Recent Advance in Electrochemical C(sp2)—H Amination of Arenes[J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 871-891.
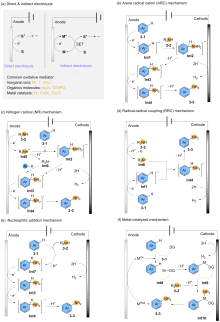

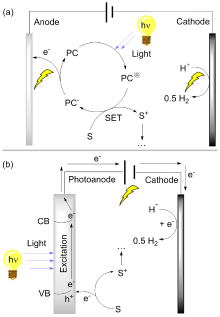
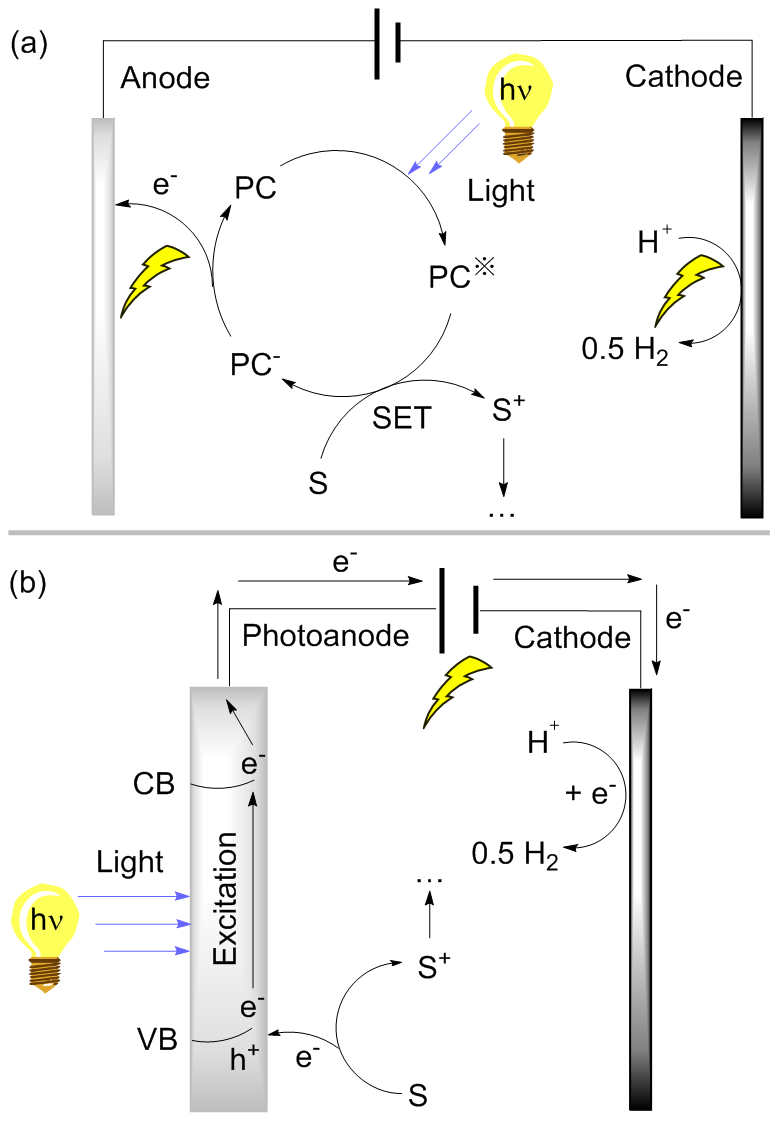
| [1] |
Amini B.; Lowenkron S. In Kirk-Othmer Encyclopedia of Chemical Technology, Ed.: Kirk-Othmer, Wiley, New York, 2003.
|
| [2] |
Rappoport Z. The Chemistry of Anilines, 1st ed., Wiley, Chichester, 2007.
|
| [3] |
Kahl T.; Schröder K.-W.; Lawrence F. R.; Marshall W. J.; Höke H.; Jäckh R.In Ullmann's Encyclopedia of Industrial Chemistry, Eds.: Elvers, B.; Bellussi, G., Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2011, p. 465-477.
|
| [4] |
Anjalin M.; Kanagathara N.; Suganthi A. R. B. Mater. Today Proc. 2020, 33, 4751.
|
| [5] |
Shuja M. H.; Shuja S. H.; Shakil F.; Ahmed I. Ann. Med. Surg. 2023, 85, 1346.
doi: 10.1097/MS9.0000000000000303 |
| [6] |
Niu W.; Li L.; Liu X.; Wang N.; Liu J.; Zhou W.; Tang Z.; Chen S. J. Am. Chem. Soc. 2015, 137, 5555.
doi: 10.1021/jacs.5b02027 |
| [7] |
Vervoort J.; De Jager P. A.; Steenbergen J.; Rietjens I. M. C. M. Xenobiotica 1990, 20, 657.
pmid: 2238701 |
| [8] |
Duckett C. J.; Lindon J. C.; Walker H.; Abou-Shakra F.; Wilson I. D; Nicholson J. K. Xenobiotica 2006, 36, 59.
pmid: 16507513 |
| [9] |
MacKetta J. J. In Encyclopedia of Chemical Processing and Design, Dekker, New York, 1990.
|
| [10] |
Béchamp Reduction, In Comprehensive Organic Name Reactions and Reagents, Wiley, Hoboken, NJ, 2010, pp. 284-287.
|
| [11] |
Porter H. K. In Organic Reactions, Ed.: Denmark, S. E., Wiley, 2011, pp. 455-481, doi: 10.1002/0471264180.or074.03.
|
| [12] |
Driessen R. T.; Kamphuis P.; Mathijssen L.; Zhang R.; Van Der Ham, L. G. J.; Van Den Berg, H.; Zeeuw, A. J. Chem. Eng. Technol. 2017, 40, 838.
doi: 10.1002/ceat.v40.5 |
| [13] |
Moreno S. N.; Docampo R. Environ. Health Perspect. 1985, 64, 199.
doi: 10.1289/ehp.8564199 |
| [14] |
Goldberg I. Ber. Dtsch. Chem. Ges. 1906, 39, 1691.
doi: 10.1002/cber.v39:2 |
| [15] |
Allen C. F. H.; McKee G. H. W. Org. Synth. 1939, 19, 6.
doi: 10.15227/orgsyn.019.0006 |
| [16] |
Paul F.; Patt J.; Hartwig J. F. J. Am. Chem. Soc. 1994, 116, 5969.
doi: 10.1021/ja00092a058 |
| [17] |
Guram A. S.; Buchwald S. L. J. Am. Chem. Soc. 1994, 116, 7901.
doi: 10.1021/ja00096a059 |
| [18] |
Chan D. M. T.; Monaco K. L.; Wang R.-P.; Winters M. P. Tetrahedron Lett. 1998, 39, 2933.
doi: 10.1016/S0040-4039(98)00503-6 |
| [19] |
Lam P. Y. S.; Clark C. G.; Saubern S.; Adams J.; Winters M. P.; Chan D. M. T.; Combs A. Tetrahedron Lett. 1998, 39, 2941.
|
| [20] |
Burns N. Z.; Baran P. S.; Hoffmann R. W. Angew. Chem., Int. Ed. 2009, 48, 2854.
doi: 10.1002/anie.v48:16 |
| [21] |
Louillat M.-L.; Patureau F. W. Chem. Soc. Rev. 2014, 43, 901.
doi: 10.1039/C3CS60318K |
| [22] |
Park Y.; Kim Y.; Chang S. Chem. Rev. 2017, 117, 9247.
doi: 10.1021/acs.chemrev.6b00644 |
| [23] |
Yang Y.; Zhang D.; Vessally E. Top. Curr. Chem. 2020, 378, 37.
|
| [24] |
Beletskaya I. P.; Averin A. D. Russ. Chem. Rev. 2021, 90, 1359.
doi: 10.1070/RCR4999 |
| [25] |
Feng Y.-L.; Shi B.-F. Chin. J. Org. Chem. 2021, 41, 3753. (in Chinese)
doi: 10.6023/cjoc202104004 |
|
( 冯亚岚, 史炳锋, 有机化学, 2021, 41, 3753.)
doi: 10.6023/cjoc202104004 |
|
| [26] |
Ravindar L.; Hasbullah S. A.; Hassan N. I.; Qin H. Eur. J. Org. Chem. 2022, 31, e202200596.
|
| [27] |
Krishna Rao M. V.; Kareem S.; Vali S. R.; Subba Reddy B. V. Org. Biomol. Chem. 2023, 21, 8426.
doi: 10.1039/D3OB01160G |
| [28] |
Boursalian G. B.; Ngai M. Y.; Hojczyk K. N.; Ritter T. J. Am. Chem. Soc. 2013, 135, 13278.
doi: 10.1021/ja4064926 pmid: 23998528 |
| [29] |
Roy S.; Panja S.; Sahoo S. R.; Chatterjee S.; Maiti D. Chem. Soc. Rev. 2023, 52, 2391.
doi: 10.1039/D0CS01466D |
| [30] |
Muñiz K. Acc. Chem. Res. 2018, 51, 1507.
doi: 10.1021/acs.accounts.8b00137 |
| [31] |
Kärkäs M. D. Chem. Soc. Rev. 2018, 47, 5786.
doi: 10.1039/C7CS00619E |
| [32] |
Liu C.; Liu J.; Li W.; Lu H.; Zhang Y. Org. Chem. Front. 2023, 10, 5309.
doi: 10.1039/D3QO01159C |
| [33] |
Zhang H.; Lei A. Synthesis 2019, 51, 83.
doi: 10.1055/s-0037-1610380 |
| [34] |
Meng Z.; Feng C.; Xu K. Chin. J. Org. Chem. 2021, 41, 2535. (in Chinese)
doi: 10.6023/cjoc202012013 |
|
( 蒙泽银, 冯承涛, 徐坤, 有机化学, 2021, 41, 2535.)
doi: 10.6023/cjoc202012013 |
|
| [35] |
Chen N.; Xu H. Green Synth. Catal. 2021, 2, 165.
|
| [36] |
Wang H.; Gao X.; Lv Z.; Abdelilah T.; Lei A. Chem. Rev. 2019, 119, 6769.
doi: 10.1021/acs.chemrev.9b00045 |
| [37] |
Lu L.; Shi R.; Lei A. Trends Chem. 2022, 4, 179.
doi: 10.1016/j.trechm.2021.12.008 |
| [38] |
Cavedon C.; Seeberger P. H.; Pieber B. Eur. J. Org. Chem. 2020, 2020, 1379.
doi: 10.1002/ejoc.v2020.10 |
| [39] |
Singh S.; Roy V. J.; Dagar N.; Sen P. P.; Roy S. R. Adv. Synth. Catal. 2021, 363, 937.
doi: 10.1002/adsc.v363.4 |
| [40] |
Kwon K.; Simons R. T.; Nandakumar M.; Roizen J. L. Chem. Rev. 2022, 122, 2353.
doi: 10.1021/acs.chemrev.1c00444 |
| [41] |
Holmberg-Douglas N.; Nicewicz D. A. Chem. Rev. 2022, 122, 1925.
doi: 10.1021/acs.chemrev.1c00311 |
| [42] |
Chan C.; Chow Y.; Yu W. Synthesis 2020, 52, 2899.
|
| [43] |
Frontana-Uribe B. A.; Little R. D.; Ibanez J. G.; Palma A.; Vasquez-Medrano R. Green Chem. 2010, 12, 2099.
doi: 10.1039/c0gc00382d |
| [44] |
Luca O. R.; Gustafson J. L.; Maddox S. M.; Fenwick A. Q.; Smith D. C. Org. Chem. Front. 2015, 2, 823.
doi: 10.1039/C5QO00075K |
| [45] |
Yan M.; Kawamata Y.; Baran P. S. Chem. Rev. 2017, 117, 13230.
doi: 10.1021/acs.chemrev.7b00397 |
| [46] |
Wiebe A.; Gieshoff T.; Möhle S.; Rodrigo E.; Zirbes M.; Waldvogel S. R. Angew. Chem., Int. Ed. 2018, 57, 5594.
doi: 10.1002/anie.v57.20 |
| [47] |
Shida N.; Zhou Y.; Inagi S. Acc. Chem. Res. 2019, 52, 2598.
doi: 10.1021/acs.accounts.9b00337 |
| [48] |
Kingston C.; Palkowitz M. D.; Takahira Y.; Vantourout J. C.; Peters B. K.; Kawamata Y.; Baran P. S. Acc. Chem. Res. 2020, 53, 72.
doi: 10.1021/acs.accounts.9b00539 |
| [49] |
Novaes L. F. T.; Liu J.; Shen Y.; Lu L.; Meinhardt J. M.; Lin S. Chem. Soc. Rev. 2021, 50, 7941.
doi: 10.1039/d1cs00223f pmid: 34060564 |
| [50] |
Feng T.; Wang S.; Qiu Y. Synlett 2022, 33, 1582.
doi: 10.1055/a-1828-1217 |
| [51] |
Ogibin Y. N.; Elinson M. N.; Nikishin G. I. Russ. Chem. Rev. 2009, 78, 89.
doi: 10.1070/RC2009v078n02ABEH003886 |
| [52] |
Francke R.; Little R. D. Chem. Soc. Rev. 2014, 43, 2492.
doi: 10.1039/c3cs60464k pmid: 24500279 |
| [53] |
Tay N. E. S.; Lehnherr D.; Rovis T. Chem. Rev. 2022, 122, 2487.
doi: 10.1021/acs.chemrev.1c00384 |
| [54] |
Mruthunjaya A. K. V.; Torriero A. A. J. Molecules 2023, 28, 471.
doi: 10.3390/molecules28020471 |
| [55] |
Roth H.; Romero N.; Nicewicz D. Synlett 2015, 27, 714.
doi: 10.1055/s-00000083 |
| [56] |
Wawzonek S.; McIntyre T. W. J. Electrochem. Soc. 1967, 114, 1025.
doi: 10.1149/1.2424177 |
| [57] |
Dvořák V.; Němec I.; Zýka J. Microchem. J. 1967, 12, 99.
doi: 10.1016/0026-265X(67)90012-4 |
| [58] |
Paduszek B.; Kalinowski M. K. Electrochim. Acta 1983, 28, 639.
doi: 10.1016/0013-4686(83)85057-9 |
| [59] |
Loveland J. W.; Dimeler G. R. Anal. Chem. 1961, 33, 1196.
doi: 10.1021/ac60177a022 |
| [60] |
O’Donnell, J. F.; Mann, C. K. J. Electroanal. Chem. Interfacial Electrochem. 1967, 13, 157.
doi: 10.1016/0022-0728(67)80108-6 |
| [61] |
Merkel P. B.; Luo P.; Dinnocenzo J. P.; Farid S. J. Org. Chem. 2009, 74, 5163.
doi: 10.1021/jo9011267 pmid: 19588891 |
| [62] |
Kita Y.; Tohma H.; Hatanaka K.; Takada T.; Fujita S.; Mitoh S.; Sakurai H.; Oka S. J. Am. Chem. Soc. 1994, 116, 3684.
doi: 10.1021/ja00088a003 |
| [63] |
Ischay M. A.; Yoon T. P. Eur. J. Org. Chem. 2012, 2012, 3359.
doi: 10.1002/ejoc.v2012.18 |
| [64] |
Yi H.; Zhang G.; Wang H.; Huang Z.; Wang J.; Singh A. K.; Lei A. Chem. Rev. 2017, 117, 9016.
doi: 10.1021/acs.chemrev.6b00620 |
| [65] |
Cui H.-L. Org. Biomol. Chem. 2020, 18, 2975.
doi: 10.1039/D0OB00441C |
| [66] |
Pistritto V. A.; Liu S.; Nicewicz D. A. J. Am. Chem. Soc. 2022, 144, 15118.
doi: 10.1021/jacs.2c04577 pmid: 35944280 |
| [67] |
Michejda C. J. W.; Hoss P. J. Am. Chem. Soc. 1970, 92, 6298.
doi: 10.1021/ja00724a032 |
| [68] |
Danen W. C.; Neugebauer F. A. Angew. Chem. Int. Ed. Engl. 1975, 14, 783.
doi: 10.1002/anie.v14:12 |
| [69] |
Chow Y. L.; Danen W. C.; Nelsen S. F.; Rosenblatt D. H. Chem. Rev. 1978, 78, 243.
doi: 10.1021/cr60313a003 |
| [70] |
Zard S. Z. Chem. Soc. Rev. 2008, 37, 1603.
doi: 10.1039/b613443m |
| [71] |
Xiong T.; Zhang Q. Chem. Soc. Rev. 2016, 45, 3069.
doi: 10.1039/c5cs00852b pmid: 27116936 |
| [72] |
Boursalian G. B.; Ham W. S.; Mazzotti A. R.; Ritter T. Nat. Chem. 2016, 8, 810.
doi: 10.1038/nchem.2529 pmid: 27442288 |
| [73] |
Pratley C.; Fenner S.; Murphy J. A. Chem. Rev. 2022, 122, 8181.
doi: 10.1021/acs.chemrev.1c00831 pmid: 35285636 |
| [74] |
Gao W.; Li W.; Zeng C.; Tian H.; Hu L.; Little R. J. Org. Chem. 2014, 79, 9613.
doi: 10.1021/jo501736w |
| [75] |
Qiu Y.; Struwe J.; Meyer T. H.; Oliveira J. C. A.; Ackermann L. Chem.-Eur. J. 2018, 24, 12784.
doi: 10.1002/chem.v24.49 |
| [76] |
Gao X.; Wang P.; Zeng L.; Tang S.; Lei A. J. Am. Chem. Soc. 2018, 140, 4195.
doi: 10.1021/jacs.7b13049 |
| [77] |
Sauermann N.; Mei R.; Ackermann L. Angew. Chem., Int. Ed. 2018, 57, 5090.
doi: 10.1002/anie.v57.18 |
| [78] |
Zhang S.; Samanta R. C.; Sauermann N.; Ackermann L. Chem.- Eur. J. 2018, 24, 19166.
doi: 10.1002/chem.v24.72 |
| [79] |
Kathiravan S.; Suriyanarayanan S.; Nicholls I. A. Org. Lett. 2019, 21, 1968.
doi: 10.1021/acs.orglett.9b00003 pmid: 30785289 |
| [80] |
Yang Q.; Wang X.; Lu J.; Zhang L.; Fang P.; Mei T. J. Am. Chem. Soc. 2018, 140, 11487.
doi: 10.1021/jacs.8b07380 |
| [81] |
Tang S.; Wang S.; Liu Y.; Cong H.; Lei A. Angew. Chem. Int. Ed. 2018, 57, 4737.
doi: 10.1002/anie.v57.17 |
| [82] |
Liu K.; Tang S.; Wu T.; Wang S.; Zou M.; Cong H.; Lei A. Nat. Commun. 2019, 10, 639.
doi: 10.1038/s41467-019-08414-8 |
| [83] |
Wu Y.; Jiang S.; Song R.; Li J. Chem. Commun. 2019, 55, 4371.
doi: 10.1039/C9CC01332F |
| [84] |
Chen S.; Li Y.; Xiang S.; Li S.; Tan B. Chem. Commun. 2021, 57, 8512.
doi: 10.1039/D1CC03276C |
| [85] |
Feng C.; Liu X.; She Y.; Shen Z.; Li M. Chin. Chem. Lett. 2023, 34, 107935.
doi: 10.1016/j.cclet.2022.107935 |
| [86] |
Wang Z.; Cheng Q.; Peng R.; Yan P.; Zeng R.; Tian W.; Pan B.; Gu J.; Li Y.; Ouyang Q. J. Org. Chem. 2022, 87, 4742.
doi: 10.1021/acs.joc.2c00031 |
| [87] |
Zincke Th.; Heuser G.; Möller W. Justus Liebigs Ann. Chem. 1904, 333, 296.
doi: 10.1002/jlac.v333:2/3 |
| [88] |
Ritter J. J.; Kalish J. J. Am. Chem. Soc. 1948, 70, 4048.
doi: 10.1021/ja01192a023 |
| [89] |
Lund H.; Tegnér C.; Takman B. Acta Chem. Scand. 1957, 11, 1323.
doi: 10.3891/acta.chem.scand.11-1323 |
| [90] |
Shine H. J.; Silber J. J.; Bussey R. J.; Okuyama T. J. Org. Chem. 1972, 37, 2691.
doi: 10.1021/jo00982a014 |
| [91] |
Blackburn G. M.; Will J. P. J. Chem. Soc., Chem. Commun. 1974, 67.
|
| [92] |
Ruhlmann L.; Schulz A.; Giraudeau A.; Messerschmidt C.; Fuhrhop J. H. J. Am. Chem. Soc. 1999, 121, 6664.
doi: 10.1021/ja984404r |
| [93] |
Li Y.; Kamata K.; Kawai T.; Abe J.; Iyoda T. J. Chem. Soc., Perkin 1 2002, 1135.
|
| [94] |
Li Y.; Asaoka S.; Yamagishi T.; Iyoda T. Electrochemistry 2004, 72, 171.
doi: 10.5796/electrochemistry.72.171 |
| [95] |
Morofuji T.; Shimizu A.; Yoshida J. Angew. Chem., Int. Ed. 2012, 51, 7259.
doi: 10.1002/anie.v51.29 |
| [96] |
Morofuji T.; Shimizu A.; Yoshida J. J. Am. Chem. Soc. 2013, 135, 5000.
doi: 10.1021/ja402083e pmid: 23510504 |
| [97] |
Herold S.; Möhle S.; Zirbes M.; Richter F.; Nefzger H.; Waldvogel S. R. Eur. J. Org. Chem. 2016, 2016, 1274.
|
| [98] |
Möhle S.; Herold S.; Richter F.; Nefzger H.; Waldvogel S. R. ChemElectroChem 2017, 4, 2196.
doi: 10.1002/celc.v4.9 |
| [99] |
Wesenberg L. J.; Herold S.; Shimizu A.; Yoshida J.; Waldvogel S. R. Chem.-Eur. J. 2017, 23, 12096.
doi: 10.1002/chem.201701979 pmid: 28605084 |
| [100] |
Strekalova S.; Kononov A.; Rizvanov I.; Budnikova Y. RSC Adv. 2021, 11, 37540.
doi: 10.1039/d1ra07650g pmid: 35496383 |
| [101] |
Taily I. M.; Saha D.; Banerjee P. Org. Lett. 2022, 24, 2310.
doi: 10.1021/acs.orglett.2c00439 |
| [102] |
Fu Y.; Zhang L.; Sun M.; Cao L.; Yang L.; Cheng R.; Ma Y.; Ye J. Eur. J. Org. Chem. 2023, 26, e202300553.
doi: 10.1002/ejoc.v26.35 |
| [103] |
Morofuji T.; Shimizu A.; Yoshida J. J. Am. Chem. Soc. 2014, 136, 4496.
doi: 10.1021/ja501093m pmid: 24625055 |
| [104] |
Morofuji T.; Shimizu A.; Yoshida J. J. Am. Chem. Soc. 2015, 137, 9816.
doi: 10.1021/jacs.5b06526 pmid: 26225441 |
| [105] |
De Robillard G.; Makni O.; Cattey H.; Andrieu J.; Devillers C. H. Green Chem. 2015, 17, 4669.
doi: 10.1039/C5GC01142F |
| [106] |
Yu Y.; Yuan Y.; Liu H.; He M.; Yang M.; Liu P.; Yu B.; Dong X.; Lei A. Chem. Commun. 2019, 55, 1809.
doi: 10.1039/C8CC09899A |
| [107] |
Wang J.-H.; Lei T.; Nan X.-L.; Wu H.-L.; Li X.-B.; Chen B.; Tung C.-H.; Wu L.-Z. Org. Lett. 2019, 21, 5581.
doi: 10.1021/acs.orglett.9b01910 |
| [108] |
Sun M.; Zhou Y.; Li L.; Wang L.; Ma Y.; Li P. Org. Chem. Front. 2021, 8, 754.
doi: 10.1039/D0QO01088J |
| [109] |
Buglioni L.; Beslać M.; Noël T. J. Org. Chem. 2021, 86, 16195.
doi: 10.1021/acs.joc.1c01409 |
| [110] |
Zhou N.; Zhao J.; Sun C.; Lai Y.; Ruan Z.; Feng P. J. Org. Chem. 2021, 86, 16059.
doi: 10.1021/acs.joc.1c01271 |
| [111] |
Xu H.-C.; Campbell J. M.; Moeller K. D. J. Org. Chem. 2014, 79, 379.
doi: 10.1021/jo402623r |
| [112] |
Hu X.; Zhang G.; Nie L.; Kong T.; Lei A. Nat. Commun. 2019, 10, 5467.
doi: 10.1038/s41467-019-13524-4 |
| [113] |
Zhang Y.; Lin Z.; Ackermann L. Chem.-Eur. J. 2021, 27, 242.
doi: 10.1002/chem.202004229 pmid: 33085807 |
| [114] |
Peng X.; Zhao J.; Ma G.; Wu Y.; Hu S.; Ruan Z.; Feng P. Green Chem. 2021, 23, 8853.
doi: 10.1039/D1GC02821A |
| [115] |
Puthanveedu M.; Khamraev V.; Brieger L.: Strohmann C.; Antonchick A. P. Chem.-Eur. J. 2021, 27, 8008.
doi: 10.1002/chem.202100960 pmid: 33931904 |
| [116] |
Zhang Y.-Z.; Mo Z.-Y.; Wang H.-S.; Wen X.-A.; Tang H.-T.; Pan Y.-M. Green Chem. 2019, 21, 3807.
doi: 10.1039/C9GC01201J |
| [117] |
Luo M.-J.; Ouyang X.-H.; Zhu Y.-P.; Li Y.; Li J.-H. Green Chem. 2021, 23, 9024.
doi: 10.1039/D1GC02922C |
| [118] |
Wang H.; Zheng Y.; Xu H.; Zou J.; Jin C. Front. Chem. 2022, 10, 950635.
doi: 10.3389/fchem.2022.950635 |
| [119] |
Morofuji T.; Shimizu A.; Yoshida J. Chem.-Eur. J. 2015, 21, 3211.
doi: 10.1002/chem.201406398 pmid: 25641711 |
| [120] |
Ohno Y.; Ando S.; Furusho D.; Hifumi R.; Nagata Y.; Tomita I.; Inagi S. Org. Lett. 2023, 25, 3951.
doi: 10.1021/acs.orglett.3c01341 |
| [121] |
Zhao H.; Liu Z.; Song J.; Xu H. Angew. Chem., Int. Ed. 2017, 56, 12732.
doi: 10.1002/anie.v56.41 |
| [122] |
Zhao H.; Xu P.; Song J.; Xu H. Angew. Chem., Int. Ed. 2018, 57, 15153.
doi: 10.1002/anie.v57.46 |
| [123] |
Zhang S.; Li L.; Xue M.; Zhang R.; Xu K.; Zeng C. Org. Lett. 2018, 20, 3443.
doi: 10.1021/acs.orglett.8b00981 |
| [124] |
Zhang P.; Li B.; Niu L.; Wang L.; Zhang G.; Jia X.; Zhang G.; Liu S.; Ma L.; Gao W.; Qin D.; Chen J. Adv. Synth. Catal. 2020, 362, 2342.
doi: 10.1002/adsc.v362.12 |
| [125] |
Wang Q.; Zhang X.; Wang P.; Gao X.; Zhang H.; Lei A. Chin. J. Chem. 2021, 39, 143.
doi: 10.1002/cjoc.v39.1 |
| [126] |
Zhang H.; Ye Z.; Chen N.; Chen Z.; Zhang F. Green Chem. 2022, 24, 1463.
doi: 10.1039/D1GC04534B |
| [127] |
Wan H.; Li D.; Xia H.; Yang L.; Alhumade H.; Yi H.; Lei A. Chem. Commun. 2022, 58, 665.
doi: 10.1039/D1CC04656J |
| [128] |
Zhao H.; Hou Z.; Liu Z.; Zhou Z.; Song J.; Xu H. Angew. Chem., Int. Ed. 2017, 56, 587.
doi: 10.1002/anie.v56.2 |
| [129] |
Zhao H.; Zhuang J.; Xu H. ChemSusChem 2021, 14, 1692.
doi: 10.1002/cssc.v14.7 |
| [130] |
Duan Z.; Zhang L.; Zhang W.; Lu L.; Zeng L.; Shi R.; Lei A. ACS Catal. 2020, 10, 3828.
doi: 10.1021/acscatal.0c00103 |
| [131] |
Yang G.; Wang Y.; Qiu Y. Chin. J. Org. Chem. 2021, 41, 3935. (in Chinese)
doi: 10.6023/cjoc202105054 |
|
( 杨光, 王衍伟, 仇友爱, 有机化学, 2021, 41, 3935.)
doi: 10.6023/cjoc202105054 |
|
| [132] |
Li P.; Zhang T.; Mushtaq M. A.; Wu S.; Xiang X.; Yan D. Chem. Rec. 2021, 21, 841.
doi: 10.1002/tcr.v21.4 |
| [133] |
Huang H.; Steiniger K. A.; Lambert T. H. J. Am. Chem. Soc. 2022, 144, 12567.
doi: 10.1021/jacs.2c01914 pmid: 35816101 |
| [134] |
Qian L.; Shi M. Chem. Commun. 2023, 59, 3487.
doi: 10.1039/D3CC00437F |
| [135] |
Decker F.; Cattarin S. In Encyclopedia of Electrochemical Power Sources, Elsevier, Amsterdam, 2009, pp. 1-9.
|
| [136] |
Huang H.; Strater Z. M.; Rauch M.; Shee J.; Sisto T. J.; Nuckolls C.; Lambert T. H. Angew. Chem., Int. Ed. 2019, 58, 13318.
doi: 10.1002/anie.v58.38 |
| [137] |
Hou Z.; Xu H. ChemElectroChem 2021, 8, 1571.
doi: 10.1002/celc.v8.9 |
| [138] |
Wu S.; Žurauskas J.; Domański M.; Hitzfeld P. S.; Butera V.; Scott D. J.; Rehbein J.; Kumar A.; Thyrhaug E.; Hauer J.; Barham J. P. Org. Chem. Front. 2021, 8, 1132.
doi: 10.1039/D0QO01609H |
| [139] |
Huang H.; Lambert T. H. Angew. Chem., Int. Ed. 2021, 60, 11163.
doi: 10.1002/anie.v60.20 |
| [140] |
Hou Z.-W.; Yan H.; Song J.; Xu H.-C. Green Chem. 2023, 25, 7959.
doi: 10.1039/D3GC02126B |
| [141] |
Zhang L.; Liardet L.; Luo J.; Ren D.; Grätzel M.; Hu X. Nat. Catal. 2019, 2, 366.
doi: 10.1038/s41929-019-0231-9 |
| [142] |
Carey F. A.; Sundberg R. J. Advanced Organic Chemistry, Springer US, Boston, MA, 2007.
|
| [1] | Yushen Gao, Yuanyuan Gao, An'an Zhang, Lu Li, Weizhi Geng, Fenghua Zhang, Fei Li, Lantao Liu. BF3•OEt2 Mediated Intramolecular Cyclization of 2-Alkynylanilines Approach to 3-Sulfenylindoles [J]. Chinese Journal of Organic Chemistry, 2024, 44(9): 2785-2795. |
| [2] | Dianfan Liu, Jianzhi Wang, Mengqing Wang, Xiaobei Chen, Yanwei Hu, Shilei Zhang. o-Iodoarylation of Thiols Enabled by Aryne Generated from o-Diiodoarene and Sodium Hydride [J]. Chinese Journal of Organic Chemistry, 2024, 44(7): 2363-2370. |
| [3] | Qian Chen, Zhaobin Han, Kuiling Ding. Transition Metal Catalyzed Carbocycle-Selective Asymmetric Hydrogenation of Aromatic Rings [J]. Chinese Journal of Organic Chemistry, 2024, 44(7): 2063-2076. |
| [4] | Jiasheng Wang, Zeshu Wang, Weimin He, Longwu Ye. Research Progress on the Hydroamination of o-Alkynylanilines for the Synthesis of Axially Chiral Indoles [J]. Chinese Journal of Organic Chemistry, 2024, 44(6): 1786-1792. |
| [5] | Wensheng Zhang, Wei Zheng, Guoqiang Zuo, Keyou Ma, Hequan Xiao, Gaiyun Liu. One-Pot Reductive Amination/N-Acylation/Aza-Wittig Reaction for the Synthesis of 3,4-Dihydroquinazolines [J]. Chinese Journal of Organic Chemistry, 2024, 44(5): 1686-1690. |
| [6] | Yafang Zhang, Xuan Huang, Qi Lin, Haiqiong Zhong, Zhiqiang Weng, Wei Wu. Recent Progress in the Synthesis of Thiazole-Containing Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(5): 1458-1479. |
| [7] | Lan Zhou, Hong He, De-Qiao Yang, Zhong-Wei Hou, Lei Wang. Electrochemical Trifluoromethylation/Spirocyclization of N-Benzylacrylamides to Construct Trifluoromethylated 2-Azaspiro[4.5]decanes [J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 981-988. |
| [8] | Jiwei Wu, Jun He, Jingjing Wang, Lixia Li, Caiyu Xu, Jie Zhou, Zirong Li, Huajian Xu. Electrochemical Oxidation Decarboxylative Cyclization of α-Keto Acid with o-Aminobenzylamine [J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 972-980. |
| [9] | Hongbing Chen, Sijia Yang, Zhipeng Ye, Kai Chen, Haoyue Xiang, Hua Yang. Electrocatalytic Reduction of Quinolines and Ketones by Using Lewis Base-Ligated Borane as a Hydrogen Donor [J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 966-971. |
| [10] | Aman Hasil, Rui Chang, Juntao Ye. Recent Advances on C—H Functionalization via Oxidative Electrophotocatalysis [J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 728-747. |
| [11] | Shuai Lv, Gangguo Zhu, Jinzhong Yao, Hongwei Zhou. Research Progress in Preparation of Carboxylic Acids by Electrochemical Mediated Oxidative Carboxylation and Reductive Carboxylation of Carbon Dioxide [J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 780-808. |
| [12] | Jian Huang, Wenzhen Zhang. Advances in Electrochemical Cathodic Reductive Reactions Involving Carbon-Nitrogen Bonds [J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 825-839. |
| [13] | Yuanhang Chen, Jinyu He, Bo Zhang, Yanzhao Wang, Lingxuan Kong, Weifeng Qian, Na'na Wang, Wenxi Duan, Yanyan Ouyang, Cuiju Zhu, Hao Xu. Asymmetric Electrochemical Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 748-779. |
| [14] | Junyong Wang, Na Li, Jie Ke, Chuan He. Recent Advances in Electrochemical Silylation [J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 927-939. |
| [15] | Xue Sun, Tingtao Yan, Kelu Yan, Jianjing Yang, Jiangwei Wen. Electrochemical Enabled Phosphorylation of α-Diazoester to Access Phosphinic Hydrazone [J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 1013-1020. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||