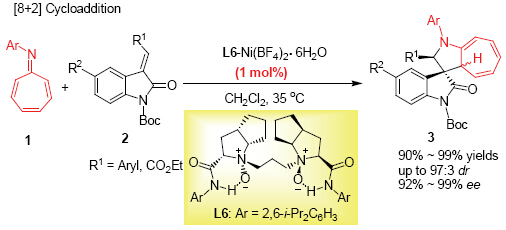
| [1] (a) Bindra, J. S. The Alkaloids, Academic Press, New York, 1973, Vol. 14, p. 84;(b) Cui, C.-B.; Kakeya, H.; Osada, H. Tetrahedron 1996, 52, 12651;(c) Cui, C.-B.; Kakeya, H.; Osada, H. J. Antibiot. 1996, 49, 832.[2] For reviews In Construction of 3,3'-Spirocyclic Oxindoles, see: (a) Marti, C.; Carreira, E. M. Eur. J. Org. Chem. 2003, 2209;(b) Galliford, C. V.; Scheidt, K. A. Angew. Chem., Int. Ed. 2007, 46, 8748;(c) Trost, B. M.; Brennan, M. K. Synthesis 2009, 3003;(d) Zhou, F.; Liu, Y.-L.; Zhou, J. Adv. Synth. Catal. 2010, 352, 1381;(e) Hong, L.; Wang, R. Adv. Synth. Catal. 2013, 355, 1023.[3] For reviews on [3+2] cycloaddition of azomethine ylides, see: (a) Stanley, L. M.; Sibi, M. P. Chem. Rev. 2008, 108, 2887;(b) Adrio, J.; Carretero, J. C. Chem. Commun. 2011, 47, 6784;(c) Pellissier, H. Tetrahedron 2012, 68, 2197.[4] For selected examples of synthesis chiral spiro[pyrrolidin- 3,3'-oxindoles] by using chiral substrates, see: (a) Sebahar, P. R.; Williams, R. M. J. Am. Chem. Soc. 2000, 122, 5666;(b) Sebahar, P. R.; Osada, H.; Usui, T.; Williams, R. M. Tetrahedron 2002, 58, 6311;(c) Onishi, T.; Sebahar, P. R.; Williams, R. M. Org. Lett. 2003, 5, 3135;(d) Lo, M. M.-C.; Neumann, C. S.; Nagayama, S.; Perlstein, E. O.; Schreiber, S. L. J. Am. Chem. Soc. 2004, 126, 16077.[5] For other efforts on the asymmetric synthesis of spiro[pyrrolidin-3,3'-oxindoles], see: (a) Overman, L. E.; Rosen, M. D. Angew. Chem., Int. Ed. 2000, 39, 4596;(b) Trost, B. M.; Brennan, M. K. Org. Lett. 2006, 8, 2027.[6] Chen, X.-H.; Wei, Q.; Luo, S.-W.; Xiao, H.; Gong, L.-Z. J. Am. Chem. Soc. 2009, 131, 13819.[7] (a) Antonchick, A. P.; Gerding-Reimers, C.; Catarinella, M.; Schürmann, M.; Preut, H.; Ziegler, S.; Rauh, D.; Waldmann, H. Nat. Chem. 2010, 2, 735;(b) Antonchick, A. P.; Schuster, H.; Bruss, H.; Schürmann, M.; Preut, H.; Rauh, D.; Waldmann, H. Tetrahedron 2011, 67, 10195;(c) Liu, T.-L.; Xue, Z.-Y.; Tao, H.-Y.; Wang, C.-J. Org. Biomol. Chem. 2011, 9, 1980;(d) Awata, A.; Arai, T. Chem.-Eur. J. 2012, 18, 8278;(e) Wang, L.; Shi, X.-M.; Dong, W.-P.; Zhu, L.-P.; Wang, R. Chem. Commun. 2013, 49, 3458.[8] For selected examples on catalytic asymmetric synthesis of other spiro-oxindoles, see: (a) Trost, B. M.; Cramer, N.; Silverman, S. M. J. Am. Chem. Soc. 2007, 129, 12396;(b) Bui, T.; Syed, S.; Barbas Ⅲ, C. F. J. Am. Chem. Soc. 2009, 131, 8758;(c) Galzerano, P.; Bencivenni, G.; Pesciaioli, F.; Mazzanti, A.; Giannichi, B.; Sambri, L.; Bartoli, G.; Melchiorre, P. Chem.-Eur. J. 2009, 15, 7846;(d) Jiang, X. X.; Cao, Y. M.; Wang, Y. Q.; Liu, L. P.; Shen, F. F.; Wang, R. J. Am. Chem. Soc. 2010, 132, 15328;(e) Wang, L.-L.; Peng, L.; Bai, J.-F.; Huang, Q.-C.; Xu, X.-Y.; Wang, L.-X. Chem. Commun. 2010, 46, 8064;(f) Chen, W.-B.; Wu, Z.-J.; Hu, J.; Cun, L.-F.; Zhang, X.-M.; Yuan, W.-C. Org. Lett. 2011, 13, 2472;(g) Liu, Y.-K.; Nappi, M.; Arceo, E.; Vera, S.; Melchiorre, P. J. Am. Chem. Soc. 2011, 133, 15212;(h) Shen, L.-T.; Shao, P.-L.; Ye, S. Adv. Synth. Catal. 2011, 353, 1943;(i) Peng, J.; Huang, X.; Jiang, L.; Cui, H.-L.; Chen, Y.-C.;Org. Lett. 2011, 13, 4584;(j) Li, Y.-M.; Li, X.; Peng, F.-Z.; Li, Z.-Q.; Wu, S.-T.; Sun, Z.-W.; Zhang, H.-B.; Shao, Z.-H. Org. Lett. 2011, 13, 6200.[9] For reviews of higher-order cycloadditions, see: (a) Rigby, J. H. Acc. Chem. Res. 1993, 26, 579;(b) Harmata, M. Acc. Chem. Res. 2001, 34, 595;(c) Harmata, M. Adv. Synth. Catal. 2006, 348, 2297;(d) Nair, V.; Abhilash, K. G. Top. Heterocycl. Chem. 2008, 13, 173;(e) Nair, V.; Abhilash, K. G. Synlett 2008, 301;(f) Lohse, A. G.; Hsung, R. P. Chem.–Eur. J. 2011, 17, 3812;(g) Pellissier, H. Adv. Synth. Catal. 2011, 353, 189;(h) Ylijoki, K. E. O.; Stryker, J. M. Chem. Rev. 2013, 113, 2244.[10] For an example of a catalytic asymmetric [6+2] cycloaddition with fulvenes as 6π components, see: (a) Hayashi, Y.; Gotoh, H.; Honma, M.; Sankar, K.; Kumar, I.; Ishikawa, H.; Konno, K.; Yui, H.; Tsuzuki, S.; Uchimaru, T. J. Am. Chem. Soc. 2011, 133, 20175; For examples of catalytic asymmetric [6+3] cycloadditions with fulvenes as 6π components, see:(b) Potowski, M.; Bauer, J. O.; Strohmann, C.; Antonchick, A. P.; Waldmann, H. Angew. Chem., Int. Ed. 2012, 51, 9512;(c) He, Z.-L.; Teng, H.-L.; Wang, C.-J. Angew. Chem., Int. Ed. 2013, 52, 2934.[11] For examples of catalytic asymmetric [5+2] cycloadditions, see: (a) Wender, P. A.; Haustedt, L. O.; Lim, J.; Love, J. A.; Williams, T. J.; Yoon, J.-Y. J. Am. Chem. Soc. 2006, 128, 6302;(b) Shintani, R.; Nakatsu, H.; Takatsu, K.; Hayashi, T. Chem.-Eur. J. 2009, 15, 8692.[12] For examples of catalytic asymmetric [4+3] cycloadditions, see: (a) Harmata, M.; Ghosh, S. K.; Hong, X.; Wacharasindhu, S.; Kirchhoefer, P. J. Am. Chem. Soc. 2003, 125, 2058;(b) Huang, J.;Hsung, R. P. J. Am. Chem. Soc. 2005, 127, 50;(c) Dai, X.; Davies, H. M. L. Adv. Synth. Catal. 2006, 348, 2449;(d) Reddy, R. P.; Davies, H. M. L. J. Am. Chem. Soc. 2007, 129, 10312;(e) Gulías, M.; Durán, J.; López, F.; Castedo, L.; Mascareñas, J. L. J. Am. Chem. Soc. 2007, 129, 11026;(f) Shintani, R.; Murakami, M.; Hayashi, T. J. Am. Chem. Soc. 2007, 129, 12356;(g) Schwartz, B. D.; Denton, J. R.; Lian, Y.; Davies, H. M. L.; Williams, C. M. J. Am. Chem. Soc. 2009, 131, 8329;(h) Shintani, R.; Murakami, M.; Tsuji, T.; Tanno, H.; Hayashi, T. Org. Lett. 2009, 11, 5642;(i) Alonso, I.; Faustino, H.; López, F.; Mascareñas, J. L. Angew. Chem., Int. Ed. 2011, 50, 11496.[13] For examples of [8+2] cycloadditions with dienylisobenzofurans as 8π components, see: (a) Luo, Y.; Herndon, J. W.; Cervantes-Lee, F. J. Am. Chem. Soc. 2003, 125, 12720;(b) Zhang, L.; Wang, Y.; Buckingham, C.; Herndon, J. W. Org. Lett. 2005, 7, 1665;(c) Chen, Y.; Ye, S.; Jiao, L.; Liang, Y.; Sinha-Mahapatra, D. K.; Herndon, J. W.; Yu, Z.-X. J. Am. Chem. Soc. 2007, 129, 10773;(d) Roy, P.; Ghorai, B. K. Tetrahedron Lett. 2011, 52, 5668.[14] For examples of [8+2] cycloadditions with indolizines as 8π components, see: (a) Godfrey, J. C. J. Org. Chem. 1959, 24, 581;(b) Galbraith, A.; Small, T.; Barnes, R. A.; Boekelheide, V. J. Am. Chem. Soc. 1961, 83, 453;(c) Aginagalde, M.; Vara, Y.; Arrieta, A.; Zangi, R.; Cebolla, V. L.; Delgado-Camón, A.; Cossío, F. P. J. Org. Chem. 2010, 75, 2776.[15] For examples of [8+2] cycloadditions with benzothietes as 8π components, see: (a) Kanakarajan, K.; Meier, H. J. Org. Chem. 1983, 48, 881;(b) Meier, H.; Mayer, A. Angew. Chem., Int. Ed. Engl. 1994, 33, 465;(c) Meier, H.; Rose, B.; Schollmeyer, D. Liebigs Ann. 1997, 1173.[16] For examples of [8+2] cycloadditions with heptafulvenes as 8π components, see: (a) Doering, W. von E.; Wiley, D. W. Tetrahedron 1960, 11, 183;(b) Liu, C.-Y.; Mareda, J.; Houk, K. N.; Fronczek, F. R. J. Am. Chem. Soc. 1983, 105, 6714;(c) Liu, C.-Y.; Shie, H.-Y.; Chen, S.-Y.; You, C.-Y.; Wang, W.-C.; Hua, L.-N.; Yang, H.-J.; Tseng, C.-M. Tetrahedron 1997, 53, 17275.[17] For an example of a catalytic asymmetric [4+2] cycloaddition with tropones as 4π components, see: (a) Li, P.; Yamamoto, H. J. Am. Chem. Soc. 2009, 131, 16628; For examples of catalytic asymmetric [6+3] cycloadditions with tropones as 6π components, see:(b) Trost, B. M.; McDougall, P. J.; Hartmann, O.; Wathen, P. T. J. Am. Chem. Soc. 2008, 130, 14960;(c) Trost, B. M.; McDougall, P. J. Org. Lett. 2009, 11, 3782; For example of a catalytic asymmetric [6+4] cycloaddition with tropones as 6π components, see:(d) Rigby, J. H.; Fleming, M. Tetrahedron Lett. 2002, 43, 8643.[18] Xie, M. S.; Liu, X. H.; Wu, X. X.; Cai, Y. F.; Lin, L. L.; Feng, X. M. Angew. Chem., Int. Ed. Engl. 2013, 52, 5604.[19] For examples of tropone as the 8π component, see: (a) Kumar, K.; Kapur, A.; Ishar, M. P. S. Org. Lett. 2000, 2, 787;(b) Okamoto, J.; Yamabe, S.; Minato, T.; Hasegawa, T.; Machiguchi, T. Helv. Chim. Acta 2005, 88, 1519;(c) Nair, V.; Poonoth, M.; Vellalath, S.; Suresh, E.; Thirumalai, R. J. Org. Chem. 2006, 71, 8964.[20] For examples of tropothione as the 8π component, see: (a) Machiguchi, T.; Hasegawa, T.; Otani, H.; Ishii, Y. J. Chem. Soc., Chem. Commun. 1987, 1375;(b) Machiguchi, T. Tetrahedron 1995, 51, 1133;(c) Nair, V.; Abhilash, K. G.; Suresh, E. Tetrahedron Lett. 2006, 47, 9329; (d) Rivero, A. R.; Fernández, I.; Sierra, M. A. J. Org. Chem. 2012, 77, 6648.[21] For examples of azaheptafulvenes as 8π components see: (a) Yamamoto, K.; Kajigaeshi, S.; Kanemasa, S. Chem. Lett. 1977, 85;(b) Yamamoto, K.; Kajigaeshi, S.; Kanemasa, S. Chem. Lett. 1977, 91;(c) Truce, W. E.; Shepherd, J. P. J. Am. Chem. Soc. 1977, 99, 6453;(d) Nair, V.; Abhilash, K. G. Tetrahedron Lett. 2006, 47, 8707;(e) Lage, M. L.; Fernández, I.; Sierra, M. A.; Torres, M. R. Org. Lett. 2011, 13, 2892.[22] (a) Sanechika, K.; Kajigaeshi, S.; Kanemasa, S. Chem. Lett. 1977, 861;(b) Ishizu, T.; Harano, K.; Yasuda, M.; Kanematsu, K. J. Org. Chem. 1981, 46, 3630;(c) Hayakawa, K.; Nishiyama, H.; Kanematsu, K. J. Org. Chem. 1985, 50, 512;(d) Barluenga, J.; García-Rodríguez, J.; Martínez, S.; Suárez-Sobrino, á. L.; Tomás, M. Chem.-Asian J. 2008, 3, 767;(e) Barluenga, J.; García-Rodríguez, J.; Suárez-Sobrino, á. L.; Tomás, M. Chem.-Eur. J. 2009, 15, 8800.[23] For selected examples using chiral N,N'-dioxide ligands, see: (a) Liu, X. H.; Lin, L. L.; Feng, X. M. Acc. Chem. Res. 2011, 44, 574;(b) Hassner, A.; Namboothiri, I. In Organic Syntheses Based on Name Reactions, 3rd ed., Elsevier, Oxford, 2011, 408;(c) Zheng, K.; Lin, L. L.; Feng, X. M. Acta Chim. Sinica 2012, 70, 1785. (郑柯, 林丽丽, 冯小明, 化学学报, 2012, 70, 1785.)(d) Zhao, J. N.; Liu, X. H.; Luo, W. W.; Xie, M. S.; Lin, L. L.; Feng, X. M. Angew. Chem., Int. Ed. Engl. 2013, 52, 3473. |