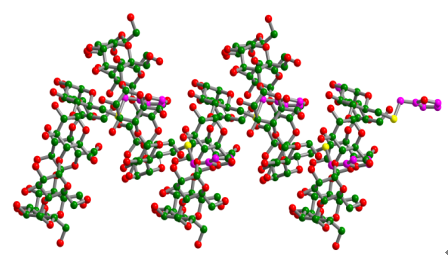
| [1] Liu, Y.; Shi, J.; Chen, Y.; Ke, C.-F. Angew. Chem., Int. Ed. 2008, 47, 7293. [2] Zhang, Z.-J.; Zhang, H.-Y.; Wang, H.; Liu, Y. Angew. Chem., Int. Ed. 2011, 50, 10834. [3] Guo, D.-S.; Wang, K.; Wang, Y.-X.; Liu, Y. J. Am. Chem. Soc. 2012, 134, 10244. [4] Zhang, Z.; Luo, Y.; Chen, J.; Dong, S.; Yu, Y.; Ma, Z.; Huang, F. Angew. Chem., Int. Ed. 2011, 50, 1397. [5] Dong, S.; Luo, Y.; Yan, X.; Zheng, B.; Ding, X.; Yu, Y.; Ma, Z.; Zhao, Q.; Huang, F. Angew. Chem., Int. Ed. 2011, 50, 1905. [6] Yan, X.-Z.; Wang, F.; Zheng, B.; Huang, F.-H. Chem. Soc. Rev. 2012, 41, 6042. [7] Liu, Y.; Zhao, Y.-L.; Zhang, H.-Y.; Song, H.-B. Angew. Chem., Int. Ed. Engl. 2003, 42, 3260. [8] Liu, Y.; Yu, Z.-L.; Zhang, Y.-M.; Guo, D.-S.; Liu, Y.-P. J. Am. Chem. Soc. 2008, 130, 10431. [9] Duan, W.-S.; Liu, B.; Sun, Z.-G.; Yang, B.-S. Acta Chim. Sinica 2011, 69, 1789. (段文胜, 刘斌, 孙占国, 杨斌盛, 化学学报, 2011, 69, 1789.) [10] Shen, H.-M.; Ji, H.-B. Chin. J. Org. Chem. 2012, 32, 975. (沈海民, 纪红兵, 有机化学, 2012, 32, 975.) [11] Li, S.-J.; Zhang, X.-J.; Liang, H.-Y.; Wang, X.-R. Acta Chim. Sinica 2012, 70, 1031. (李姝静, 张小军, 梁海燕, 王心蕊, 化学学报, 2012, 70, 1031.) [12] Zhu, L.-L.; Yan, H.; Nguyen, K.-T.; Tian, H.; Zhao, Y.-L. Chem. Commun. 2012, 48, 4290. [13] Ke, C.-F.; Yang, C.; Mori, T.; Wada, T.; Liu, Y.; Inoue, Y. Angew. Chem., Int. Ed. 2009, 48, 6675. [14] Liu, Y.; Ke, C.-F.; Zhang, H.-Y.; Cui, J.; Ding, F. J. Am. Chem. Soc. 2008, 130, 600. [15] Wang, H.-S.; Peng, J.-T.; Wei, J.-P.; Jiang, A. Acta Chim. Sinica 2012, 70, 1355. (王怀松, 彭江涛, 魏纪平, 姜安, 化学学报, 2012, 70, 1355.) [16] Tang, K.-W.; Li, H.-J.; Liu, Y.-B. Acta Chim. Sinica 2011, 69, 2696. (唐课文, 李洪建, 刘永兵, 化学学报, 2011, 69, 2696.) [17] Yang, C.; Ke, C.-F.; Liang, W.-T.; Fukuhara, G.; Mori, T.; Liu, Y. J. Am. Chem. Soc. 2011, 133, 13786. [18] Du, P.; Chen, G.-S.; Jiang, M. Sci. China, Ser. B-Chem. 2012, 42, 723. (杜萍, 陈国颂, 江明, 中国科学B辑: 化学, 2012, 42, 723.) [19] Zhou, Y.-H.; Mao, Z.-W. Sci. China, Ser. B-Chem. 2009, 39, 289. (周映华, 毛宗万, 中国科学B辑: 化学, 2009, 39, 289.) [20] Yan, H.; Teh, C.; Sreejith, S.; Zhu, L.-L.; Kwok, A.; Fang, W.-Q.; Ma, X.; Nguyen, K. T.; Korzh, V.; Zhao, Y.-L. Angew. Chem., Int. Ed. 2012, 51, 8373. [21] Zhu, L.-L.; Yan, H.; Zhao, Y.-L. Int. J. Mol. Sci. 2012, 13, 10132. [22] Chen, Y.; Liu, Y. Chin. J. Org. Chem. 2012, 32, 805. (陈湧, 刘育, 有机化学, 2012, 32, 805.) [23] Zhang, H.-C.; Liu, Z.-N.; An, W.; Hao, A.-Y.; Sun, L.-Z. Chin. J. Org. Chem. 2010, 30, 1279. (张华承, 刘召娜, 安伟, 郝爱友, 孙立臻, 有机化学, 2010, 30, 1279.) [24] Jiang, J.-Q.; Chen, X.; Yin, M.; Wang, Z.-P.; Liu, X.-Y.; Chen, M.-Q. Acta Chim. Sinica 2012, 70, 525. (江金强, 陈欣, 殷明, 王周平, 刘晓亚, 陈明清, 化学学报, 2012, 70, 525.) [25] Hirotsu, K.; Higuchi, T.; Fujita, K.; Ueda, T.; Shinoda, A.; Imoto, T.; Tabushi, I. J. Org. Chem. 1982, 47, 1143. [26] Harata, K. Chem. Rev. 1998, 98, 1803. [27] Kamitori, S.; Hirotsu, K.; Higuchi, T.; Fujita, K.; Yamamura, H.; Imoto, T.; Tabushi, I. J. Chem. Soc., Perkin Trans. 2 1987, 7. [28] Harata, K.; Takenaka, Y.; Yoshida, N. J. Chem. Soc., Perkin Trans. 2 2001, 1667. [29] Liu, Y.; You, C.-C.; Zhang, M.; Weng, L.-H.; Wada, T.; Inoue, Y. Org. Lett. 2000, 2, 2761. [30] Liu, Y.; Fan, Z.; Zhang, H.-Y.; Yang, Y.-W.; Ding, F.; Liu, S.-X.; Wu, X.; Wada, T.; Inoue, Y. J. Org. Chem. 2003, 68, 8345. [31] Zhao, Y.-L.; Liu, Y. Sci. China, Ser. B-Chem. 2006, 49, 230. [32] Fan, Z.; Guo, M.-J.; Dong, B.; Diao, C.-H.; Jing, Z.-L.; Chen, X. Sci. China, Ser. B-Chem. 2010, 53, 1089. [33] Li, W.-J.; Fan, Z.; Diao, C.-H.; Wang, M. Carbohydr. Res. 2012, 349, 103. [34] McAlpine, S. R.; Garcia-Garibay, M. A. J. Am. Chem. Soc. 1998, 120, 4269. |