化学学报 ›› 2022, Vol. 80 ›› Issue (5): 640-646.DOI: 10.6023/A21120614 上一篇 下一篇
所属专题: 中国科学院青年创新促进会合辑
研究论文
吕天天a,b, 马文b, 詹冬笋b, 邹燕敏b, 李继龙b, 冯美玲b,*( ), 黄小荥b
), 黄小荥b
投稿日期:2021-12-31
发布日期:2022-05-31
通讯作者:
冯美玲
作者简介:基金资助:
Tiantian Lüa,b, Wen Mab, Dongsun Zhanb, Yanmin Zoub, Jilong Lib, Meiling Fengb( ), Xiaoying Huangb
), Xiaoying Huangb
Received:2021-12-31
Published:2022-05-31
Contact:
Meiling Feng
About author:Supported by:文章分享

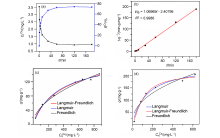
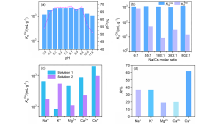
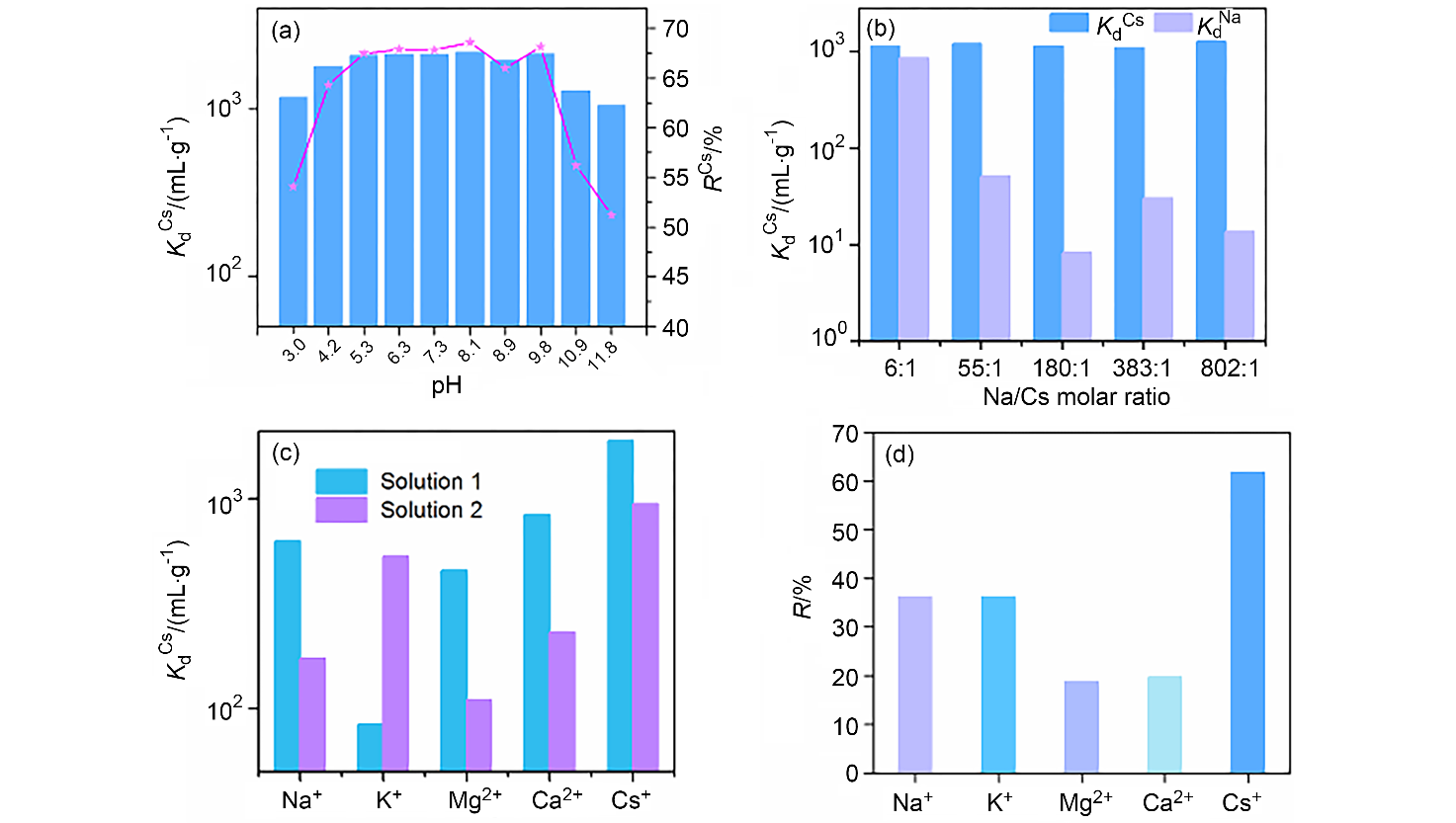
137Cs具有强放射性和较长半衰期, 一旦从核废液中泄露将对人类健康和环境造成很大危害. 由于137Cs+的高溶解性、易迁移性和废液中干扰离子的影响, 从复杂的放射性废液中有效去除137Cs+仍然是一个挑战. 本研究通过溶剂热法合成了两例新的三维微孔镧系金属-有机框架化合物(Me2NH2)0.5(H3O)0.25Na0.25Ln(OH)(stp)•0.25H2O (FJSM-LnMOF; Ln=Eu, Tb; H3stp=2-磺酸基对苯二甲酸), 它们具有良好的水稳定性和一定的耐酸碱性. FJSM-EuMOF和FJSM-TbMOF对Cs+离子吸附具有快速的动力学和高的吸附量(qmCs分别为229.25和211.28 mg/g). 它们对Cs+离子具有良好的选择性(KdCs值高达2.18×103 mL/g). 即使在Na+, K+, Mg2+, Ca2+离子干扰的情况下, 它们仍然表现出对Cs+离子的选择性吸附性能. 我们成功获得了Cs+吸附产物的单晶结构, 通过单晶结构分析结合X射线光电子能谱(XPS), 红外(IR), 扫描电镜能量色散谱(EDS)和元素分析(EA)等多种表征方法, 证实了FJSM-EuMOF对Cs+离子的吸附为离子交换的机理. 结果表明, FJSM-EuMOF对Cs+离子的高效吸附主要源于镧系金属-有机阴离子框架中有机配体上的 COO–和 $\text{SO}_{3}^{}$官能团对Cs+离子强的作用力以及通道内存在易交换的[Me2NH2]+阳离子和[H3O]+离子. 这项工作表明, 镧系金属-有机框架化合物在放射性铯的修复中具有潜在的应用价值.
吕天天, 马文, 詹冬笋, 邹燕敏, 李继龙, 冯美玲, 黄小荥. 两例新的镧系金属-有机框架化合物高效去除Cs+离子研究※[J]. 化学学报, 2022, 80(5): 640-646.
Tiantian Lü, Wen Ma, Dongsun Zhan, Yanmin Zou, Jilong Li, Meiling Feng, Xiaoying Huang. Two New Three-Dimensional Lanthanide Metal-organic Frameworks for the Highly Efficient Removal of Cs+ Ions※[J]. Acta Chimica Sinica, 2022, 80(5): 640-646.
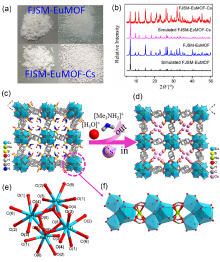


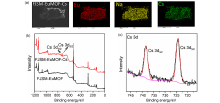
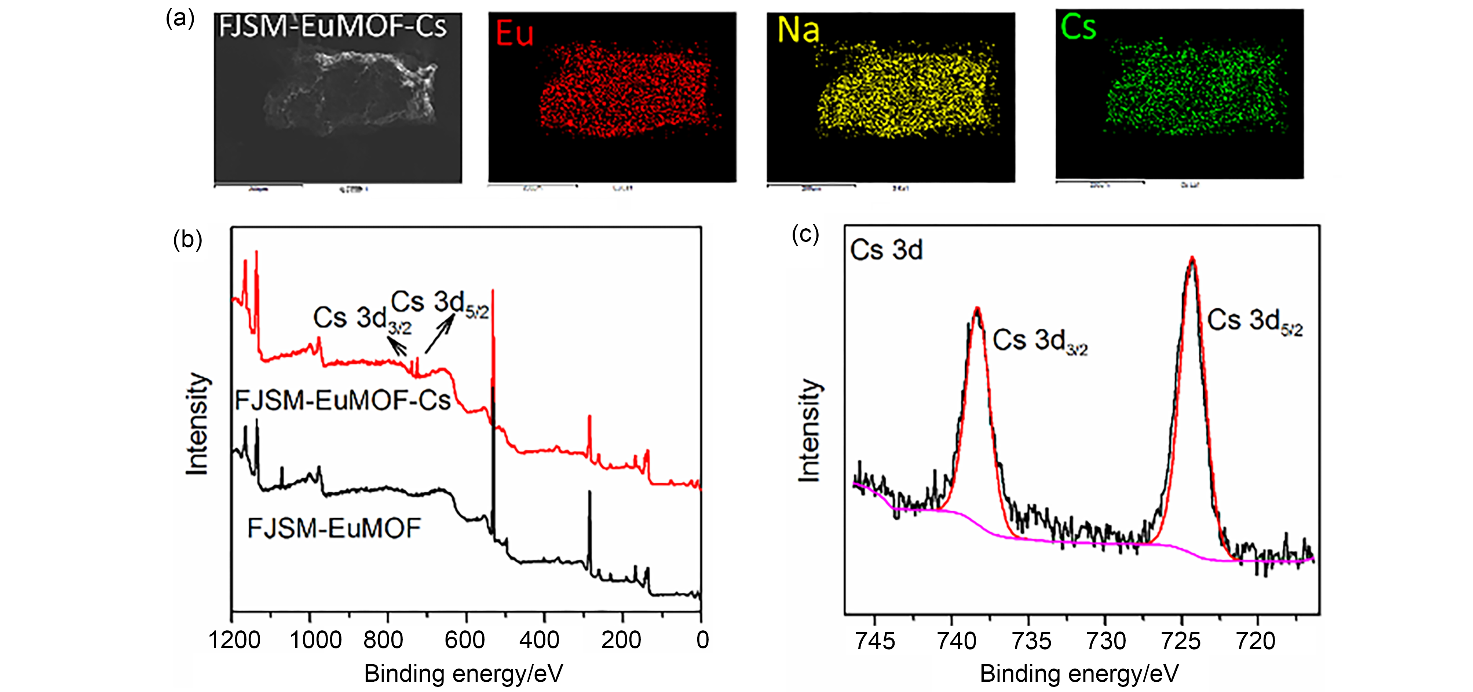
| [1] |
Wang, N.; Pang, H. W.; Yu, S. J.; Gu, P. C.; Song, S.; Wang, H. Q.; Wang, X. K. Acta Chim. Sinica 2019, 77, 143. (in Chinese)
doi: 10.6023/A18090404 |
|
(王宁, 庞宏伟, 于淑君, 顾鹏程, 宋爽, 王宏青, 王祥科, 化学学报, 2019, 77, 143.)
doi: 10.6023/A18090404 |
|
| [2] |
Rahman, R. O. A.; Ibrahium, H. A.; Hung, Y. T. Water 2011, 3, 551.
doi: 10.3390/w3020551 |
| [3] |
Smith, J. T.; Wright, S. M.; Cross, M. A.; Monte, L.; Kudelsky, A. V.; Saxen, R.; Vakulovsky, S. M.; Timms, D. N. Environ. Sci. Technol. 2004, 38, 850.
pmid: 14968873 |
| [4] |
Ivanov, Y. A.; Lewyckyj, N.; Levchuk, S. E.; Prister, B. S.; Firsakova, S. K.; Arkhipov, N. P.; Arkhipov, A. N.; Kruglov, S. V.; Alexakhin, R. M.; Sandalls, J.; Askbrant, S. J. Environ. Radioact. 1997, 35, 1.
doi: 10.1016/S0265-931X(96)00036-7 |
| [5] |
Namiki, Y.; Namiki, T.; Ishii, Y.; Koido, S.; Nagase, Y.; Tsubota, A.; Tada, N.; Kitamoto, Y. Pharm. Res. 2012, 29, 1404.
doi: 10.1007/s11095-011-0628-x |
| [6] |
Li, G. D.; Ji, G. X.; Sun, X. L.; Du, W.; Liu, W.; Wang, S. A. J. Inorg. Mater. 2019, 35, 367. (in Chinese)
|
|
(李国东, 姬国勋, 孙新利, 杜伟, 刘伟, 王殳凹, 无机材料学报, 2019, 35, 367.)
|
|
| [7] |
Buesseler, K.; Aoyama, M.; Fukasawa, M. Environ. Sci. Technol. 2011, 45, 9931.
doi: 10.1021/es202816c pmid: 22013920 |
| [8] |
Shakir, K.; Sohsah, M.; Soliman, M. Sep. Purif. Technol. 2007, 54, 373.
doi: 10.1016/j.seppur.2006.10.006 |
| [9] |
Wang, J. L.; Zhuang, S. T. Rev. Environ. Sci. Biotechnol. 2019, 18, 231.
doi: 10.1007/s11157-019-09499-9 |
| [10] |
Sinha, P. K.; Panicker, P. K.; Amalraj, R. V.; Krishnasamy, V. Waste Manage. (Oxford) 1995, 15, 149.
|
| [11] |
Ding, D. H.; Lei, Z. F.; Yang, Y. N.; Zhang, Z. Y. Chem. Eng. J. 2014, 236, 17.
doi: 10.1016/j.cej.2013.09.075 |
| [12] |
Kim, T. Y.; An, S. S.; Shim, W. G.; Lee, J. W.; Cho, S. Y.; Kim, J. H. J. Ind. Eng. Chem. 2015, 27, 260.
|
| [13] |
Yu, S. J.; Tang, H.; Zhang, D.; Wang, S. Q.; Qiu, M. Q.; Song, G.; Fu, D.; Hu, B. W.; Wang, X. K. Sci. Total Environ. 2022, 811, 152280.
doi: 10.1016/j.scitotenv.2021.152280 |
| [14] |
Wang, X. X.; Chen, L.; Wang, L.; Fan, Q. H.; Pan, D. Q.; Li, J. X.; Chi, F. T.; Xie, Y.; Yu, S. J.; Xiao, C. L.; Luo, F.; Wang, J.; Wang, X. L.; Chen, C. L.; Wu, W. S.; Shi, W. Q.; Wang, S.; Wang, X. K. Sci. China Chem. 2019, 62, 933.
doi: 10.1007/s11426-019-9492-4 |
| [15] |
Li, J.; Wang, X. X.; Zhao, G. X.; Chen, C. L.; Chai, Z. F.; Alsaedi, A.; Hayat, T.; Wang, X. K. Chem. Soc. Rev. 2018, 47, 2322.
doi: 10.1039/C7CS00543A |
| [16] |
Lysova, A. A.; Samsonenko, D. G.; Dorovatovskii, P. V.; Lazarenko, V. A.; Khrustalev, V. N.; Kovalenko, K. A.; Dybtsev, D. N.; Fedin, V. P. J. Am. Chem. Soc. 2019, 141, 17260.
doi: 10.1021/jacs.9b08322 pmid: 31584810 |
| [17] |
Dai, M.; Wang, J.; Li, L.; Wang, Q.; Liu, M.; Zhang, Y. Acta Chim. Sinica 2020, 78, 355. (in Chinese)
doi: 10.6023/A20010017 |
|
(代迷迷, 王健, 李麟阁, 王琪, 刘美男, 张跃钢, 化学学报, 2020, 78, 355.)
doi: 10.6023/A20010017 |
|
| [18] |
Guo, C.; Ma, X.; Wang, B. Acta Chim. Sinica 2021, 79, 967. (in Chinese)
doi: 10.6023/A21040173 |
|
郭彩霞, 马小杰, 王博, 化学学报, 2021, 79, 967.)
doi: 10.6023/A21040173 |
|
| [19] |
Lü, L.; Zhao, Y.; Wei, Y.; Wang, H. Acta Chim. Sinica 2021, 79, 869. (in Chinese)
doi: 10.6023/A21030099 |
|
(吕露茜, 赵娅俐, 魏嫣莹, 王海辉, 化学学报, 2021, 79, 869.)
doi: 10.6023/A21030099 |
|
| [20] |
Jin, K.; Lee, B.; Park, J. Coord. Chem. Rev. 2021, 427, 213473.
doi: 10.1016/j.ccr.2020.213473 |
| [21] |
Patra, K.; Ansari, S. A.; Mohapatra, P. K. J. Chromatogr. A 2021, 1655, 462491.
|
| [22] |
Wang, Y.; Liu, Z.; Li, Y.; Bai, Z.; Liu, W.; Wang, Y.; Xu, X.; Xiao, C.; Sheng, D.; Juan, D.; Su, J.; Chai, Z.; Albrecht-Schmitt, T. E.; Wang, S. J. Am. Chem. Soc. 2015, 137, 6144.
doi: 10.1021/jacs.5b02480 |
| [23] |
Liu, X. L.; Pang, H. W.; Liu, X. W.; Li, Q.; Zhang, N.; Mao, L.; Qiu, M. Q.; Hu, B. W.; Yang, H.; Wang, X. K. Innovation 2021, 2, 100076.
|
| [24] |
Zhang, N.; Yuan, L. Y.; Guo, W. L.; Luo, S. Z.; Chai, Z. F.; Shi, W. Q. ACS Appl. Mater. Interfaces 2017, 9, 25216.
doi: 10.1021/acsami.7b04192 |
| [25] |
Feng, Y. F.; Jiang, H.; Li, S. N.; Wang, J.; Jing, X. Y.; Wang, Y. R.; Chen, M. Colloids Surf., A 2013, 431, 87.
|
| [26] |
Rapti, S.; Sarma, D.; Diamantis, S. A.; Skliri, E.; Armatas, G. S.; Tsipis, A. C.; Hassan, Y. S.; Alkordi, M.; Malliakas, C. D.; Kanatzidis, M. G.; Lazarides, T.; Plakatouras, J. C.; Manos, M. J. J. Mater. Chem. A 2017, 5, 14707.
doi: 10.1039/C7TA04496H |
| [27] |
Aguila, B.; Banerjee, D.; Nie, Z. M.; Shin, Y.; Ma, S. Q.; Thallapally, P. K. Chem. Commun. 2016, 52, 5940.
doi: 10.1039/C6CC00843G |
| [28] |
Gao, Y. J.; Feng, M. L.; Zhang, B.; Wu, Z. F.; Song, Y.; Huang, X. Y. J. Mater. Chem. A 2018, 6, 3967.
doi: 10.1039/C7TA11208D |
| [29] |
Feng, M. L.; Sarma, D.; Gao, Y. J.; Qi, X. H.; Li, W. A.; Huang, X. Y.; Kanatzidis, M. G. J. Am. Chem. Soc. 2018, 140, 11133.
doi: 10.1021/jacs.8b07457 |
| [30] |
Tang, J. H.; Sun, H. Y.; Ma, W.; Feng, M. L.; Huang, X. Y. Chin. J. Struct. Chem. 2020, 39, 2157.
|
| [31] |
Feng, M. L.; Sarma, D.; Qi, X. H.; Du, K. Z.; Huang, X. Y.; Kanatzidis, M. G. J. Am. Chem. Soc. 2016, 138, 12578.
doi: 10.1021/jacs.6b07351 |
| [32] |
Feng, M. L.; Wang, K. Y.; Huang, X. Y. Chem. Rec. 2016, 16, 582.
doi: 10.1002/tcr.201500243 |
| [33] |
Qi, X. H.; Du, K. Z.; Feng, M. L.; Li, J. R.; Du, C. F.; Zhang, B.; Huang, X. Y. J. Mater. Chem. A 2015, 3, 5665.
doi: 10.1039/C5TA00566C |
| [34] |
Zeng, X.; Liu, Y.; Zhang, T.; Jin, J. C.; Li, J. L.; Sun, Q.; Ai, Y. J.; Feng, M. L.; Huang, X. Y. Chem. Eng. J. 2021, 420.
|
| [35] |
Liao, Y. Y.; Li, J. R.; Zhang, B.; Sun, H. Y.; Ma, W.; Jin, J. C.; Feng, M. L.; Huang, X. Y. ACS Appl. Mater. Interfaces 2021, 13, 5275.
doi: 10.1021/acsami.0c21756 |
| [36] |
Li, W. A.; Li, J. R.; Zhang, B.; Sun, H. Y.; Jin, J. C.; Huang, X. Y.; Feng, M. L. ACS Appl. Mater. Interfaces 2021, 13, 10191.
doi: 10.1021/acsami.0c22690 |
| [37] |
Li, J.; Jin, J.; Zou, Y.; Sun, H.; Zeng, X.; Huang, X.; Feng, M.; Kanatzidis, M. G. ACS Appl. Mater. Interfaces 2021, 13, 13434.
doi: 10.1021/acsami.1c01983 |
| [38] |
Sun, H. Y.; Liu, Y.; Lin, J.; Yue, Z. H.; Li, W. A.; Jin, J. C.; Sun, Q.; Ai, Y. J.; Feng, M. L.; Huang, X. Y. Angew. Chem., Int. Ed. 2020, 59, 1878.
doi: 10.1002/anie.201912040 |
| [39] |
El-Kamash, A. M. J. Hazard. Mater. 2008, 151, 432.
|
| [40] |
Mertz, J. L.; Fard, Z. H.; Malliakas, C. D.; Manos, M. J.; Kanatzidis, M. G. Chem. Mater. 2013, 25, 2116.
doi: 10.1021/cm400699r |
| [41] |
Ali, I. M.; Zakaria, E. S.; Aly, H. F. J. Radioanal. Nucl. Chem. 2010, 285, 483.
doi: 10.1007/s10967-010-0612-7 |
| [42] |
Datta, S. J.; Moon, W. K.; Choi, D. Y.; Hwang, I. C.; Yoon, K. B. Angew. Chem., Int. Ed. 2014, 53, 7203.
doi: 10.1002/anie.201402778 |
| [43] |
Park, Y.; Lee, Y. C.; Shin, W. S.; Choi, S. J. Chem. Eng. J. 2010, 162, 685.
doi: 10.1016/j.cej.2010.06.026 |
| [44] |
Naeimi, S.; Faghihian, H. Sep. Purif. Technol. 2017, 175, 255.
doi: 10.1016/j.seppur.2016.11.028 |
| [45] |
He, J.; Ma, P. F.; Wang, Y.; Li, R. H.; Yuan, X. Q.; Zhang, L. J. Non-Cryst. Solids 2016, 431, 130.
doi: 10.1016/j.jnoncrysol.2015.04.030 |
| [1] | 刘洋, 高丰琴, 马占营, 张引莉, 李午戊, 侯磊, 张小娟, 王尧宇. 一例钴基金属有机框架化合物活化过氧单硫酸盐高效降解水中亚甲基蓝研究[J]. 化学学报, 2024, 82(2): 152-159. |
| [2] | 李雅宁, 王晓艳, 唐勇. 自由基聚合的立体选择性调控★[J]. 化学学报, 2024, 82(2): 213-225. |
| [3] | 王成强, 冯超. 亲核性氟源在碳碳不饱和键选择性氟化官能化反应中的应用[J]. 化学学报, 2024, 82(2): 160-170. |
| [4] | 王志强, 苏进展. 磷酸钴修饰Cu3V2O8/ZnO光阳极的动力学特性及光电化学水分解研究[J]. 化学学报, 2024, 82(1): 26-35. |
| [5] | 黄涎廷, 韩洪亮, 肖婧, 王帆, 柳忠全. I2O5/KSCN介导的炔烃碘硫氰化反应[J]. 化学学报, 2024, 82(1): 5-8. |
| [6] | 吴宇晗, 张栋栋, 尹宏宇, 陈正男, 赵文, 匙玉华. “双碳”目标下Janus In2S2X光催化还原CO2的密度泛函理论研究[J]. 化学学报, 2023, 81(9): 1148-1156. |
| [7] | 翟彤仪, 葛畅, 钱鹏程, 周波, 叶龙武. Brønsted酸催化炔酰胺分子内氢烷氧化/Claisen重排反应★[J]. 化学学报, 2023, 81(9): 1101-1107. |
| [8] | 王海朋, 蔡文生, 邵学广. 抗冻剂抗冻机制的近红外光谱与分子模拟研究★[J]. 化学学报, 2023, 81(9): 1167-1174. |
| [9] | 杨蓉婕, 周璘, 苏彬. 基于共价有机框架修饰电极的维生素A和C的选择性检测★[J]. 化学学报, 2023, 81(8): 920-927. |
| [10] | 魏文杰, 陈文龙, 戴晓彬, 燕立唐. 生物大分子介质中的反常扩散动力学理论★[J]. 化学学报, 2023, 81(8): 967-978. |
| [11] | 张艳东, 朱守非. 环丙烷骨架膦配体的研究展望★[J]. 化学学报, 2023, 81(7): 777-783. |
| [12] | 坎比努尔•努尔买买提, 王超, 罗时玮, 阿布都热西提•阿布力克木. 电化学条件下α,α,α-三卤(氯, 溴)甲基酮类化合物的选择性脱卤反应研究[J]. 化学学报, 2023, 81(6): 582-587. |
| [13] | 刘露杰, 张建, 王亮, 肖丰收. 生物质基多元醇的多相催化选择性氢解★[J]. 化学学报, 2023, 81(5): 533-547. |
| [14] | 刘祯钰, 甘利华. 乙炔热解为富勒烯的分子动力学模拟研究[J]. 化学学报, 2023, 81(5): 502-510. |
| [15] | 徐斌, 韦秀芝, 孙江敏, 刘建国, 马隆龙. 原位合成氮掺杂石墨烯负载钯纳米颗粒用于催化香兰素高选择性加氢反应[J]. 化学学报, 2023, 81(3): 239-245. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||