化学学报 ›› 2023, Vol. 81 ›› Issue (5): 502-510.DOI: 10.6023/A23020026 上一篇 下一篇
研究论文
投稿日期:2023-02-09
发布日期:2023-04-24
基金资助:Received:2023-02-09
Published:2023-04-24
Contact:
*E-mail: ganlh@swu.edu.cn
Supported by:文章分享
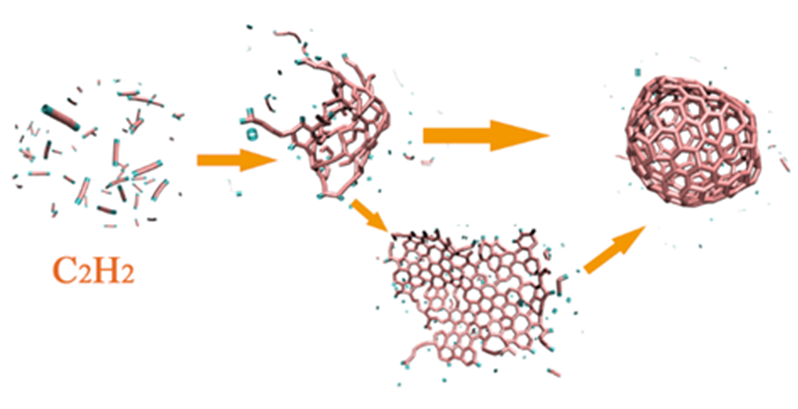
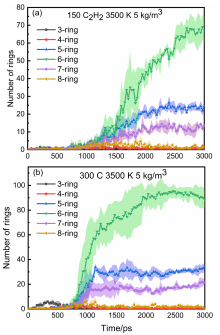
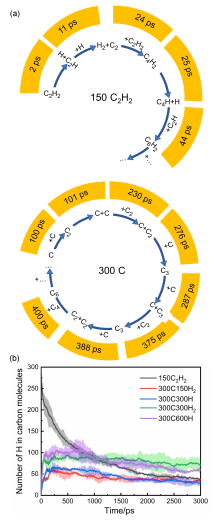
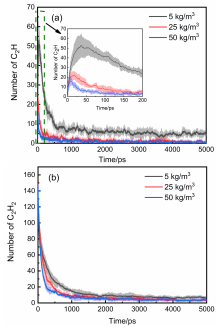
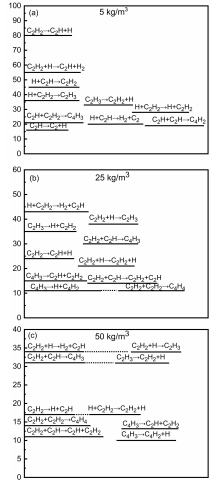
通过分子动力学模拟, 探讨了乙炔高温热解制备富勒烯的可能条件. 结果显示, 乙炔裂解可以形成富勒烯. 在裂解过程中, 乙炔热解产生的氢原子和氢分子能起到球磨碳团簇和阻止其尺寸过度增长的作用, 并促使碳笼更趋近于经典富勒烯, 暗示从炔烃制备富勒烯不需要惰性气体的协助, 从而可显著降低富勒烯的生产成本. 模拟显示, 以乙炔为反应物时, 主要有两种途径形成富勒烯. 第一种是碳链→团簇→碳笼, 多发生于低密度条件下; 第二种是碳链→团簇→碳片→碳笼, 多发生于高密度条件下. 在富勒烯形成过程中, 六元环的增长是由碳笼缺陷和键的旋转导致的. 这些结果有助于理解从炔烃热解制备富勒烯的过程, 对开发富勒烯的低成本生产方法具有重要启示意义.
刘祯钰, 甘利华. 乙炔热解为富勒烯的分子动力学模拟研究[J]. 化学学报, 2023, 81(5): 502-510.
Zhenyu Liu, Li-Hua Gan. Molecular Dynamics Simulation of Acetylene Pyrolysis into Fullerenes[J]. Acta Chimica Sinica, 2023, 81(5): 502-510.

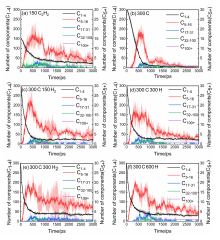


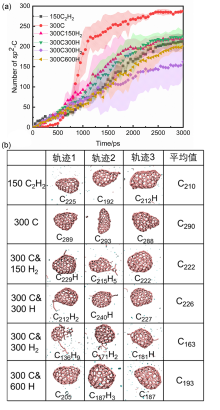

| System | N5 | N6 | N5+N6 | N3-8 | N6/N3-8 | (N5+N6)/N3-8 |
|---|---|---|---|---|---|---|
| 150 C2H2 | 24 | 67 | 91 | 108 | 0.620 | 0.843 |
| 300 C | 34 | 89 | 123 | 146 | 0.610 | 0.842 |
| 300 C&300 H | 24 | 74 | 98 | 112 | 0.661 | 0.875 |
| 300 C&300 H2 | 18 | 47 | 65 | 75 | 0.627 | 0.867 |
| 300 C&600 H | 22 | 65 | 87 | 98 | 0.663 | 0.888 |
| System | N5 | N6 | N5+N6 | N3-8 | N6/N3-8 | (N5+N6)/N3-8 |
|---|---|---|---|---|---|---|
| 150 C2H2 | 24 | 67 | 91 | 108 | 0.620 | 0.843 |
| 300 C | 34 | 89 | 123 | 146 | 0.610 | 0.842 |
| 300 C&300 H | 24 | 74 | 98 | 112 | 0.661 | 0.875 |
| 300 C&300 H2 | 18 | 47 | 65 | 75 | 0.627 | 0.867 |
| 300 C&600 H | 22 | 65 | 87 | 98 | 0.663 | 0.888 |


| 密度及编号 | 出现时间 | 组成 | 消失时间 | 组成 | 存在时长 | H/C变化 |
|---|---|---|---|---|---|---|
| 5-4 | 1.450 | C90H5 | 1.542 | C122H8 | 0.092 | 0.010 |
| 25-2 | 0.540 | C124H17 | 0.640 | C112H11 | 0.100 | -0.039 |
| 25-5 | 0.540 | C200H24 | 0.825 | C247H32 | 0.285 | 0.010 |
| 5-5 | 1.300 | C108H5 | 1.850 | C183H7 | 0.550 | -0.008 |
| 50-5 | 0.500 | C229H39 | 1.260 | C283H26 | 0.760 | -0.078 |
| 50-4 | 0.550 | C233H39 | 1.510 | C284H17 | 0.960 | -0.108 |
| 25-7 | 0.790 | C162H21 | 1.800 | C262H14 | 1.010 | -0.076 |
| 25-3 | 0.580 | C150H22 | 1.680 | C277H19 | 1.100 | -0.078 |
| 50-2 | 0.620 | C204H38 | 2.050 | C285H18 | 1.430 | -0.123 |
| 25-0 | 0.750 | C229H36 | 2.190 | C283H19 | 1.440 | -0.090 |
| 25-4 | 0.617 | C162H24 | 2.065 | C279H17 | 1.448 | -0.087 |
| 50-9 | 0.540 | C212H43 | 1.990 | C283H24 | 1.450 | -0.118 |
| 25-8 | 0.700 | C199H29 | 5.000 | C295H14 | 4.300 | -0.098 |
| 密度及编号 | 出现时间 | 组成 | 消失时间 | 组成 | 存在时长 | H/C变化 |
|---|---|---|---|---|---|---|
| 5-4 | 1.450 | C90H5 | 1.542 | C122H8 | 0.092 | 0.010 |
| 25-2 | 0.540 | C124H17 | 0.640 | C112H11 | 0.100 | -0.039 |
| 25-5 | 0.540 | C200H24 | 0.825 | C247H32 | 0.285 | 0.010 |
| 5-5 | 1.300 | C108H5 | 1.850 | C183H7 | 0.550 | -0.008 |
| 50-5 | 0.500 | C229H39 | 1.260 | C283H26 | 0.760 | -0.078 |
| 50-4 | 0.550 | C233H39 | 1.510 | C284H17 | 0.960 | -0.108 |
| 25-7 | 0.790 | C162H21 | 1.800 | C262H14 | 1.010 | -0.076 |
| 25-3 | 0.580 | C150H22 | 1.680 | C277H19 | 1.100 | -0.078 |
| 50-2 | 0.620 | C204H38 | 2.050 | C285H18 | 1.430 | -0.123 |
| 25-0 | 0.750 | C229H36 | 2.190 | C283H19 | 1.440 | -0.090 |
| 25-4 | 0.617 | C162H24 | 2.065 | C279H17 | 1.448 | -0.087 |
| 50-9 | 0.540 | C212H43 | 1.990 | C283H24 | 1.450 | -0.118 |
| 25-8 | 0.700 | C199H29 | 5.000 | C295H14 | 4.300 | -0.098 |


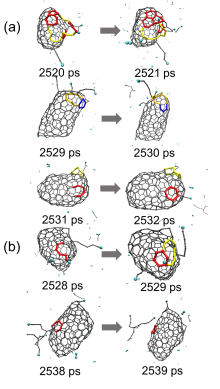

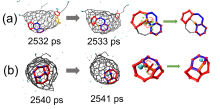
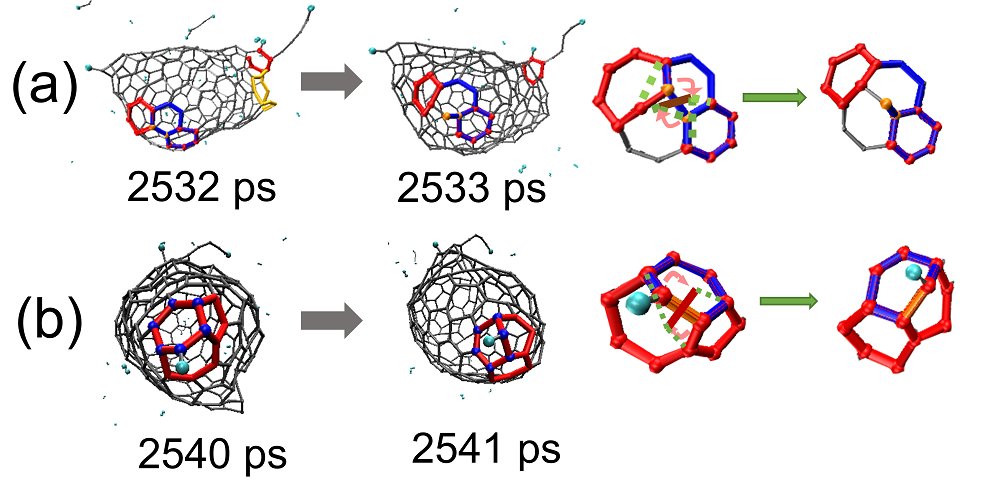
| Time/ps | δN6 | 缺口收缩/扩大 | 碳链 | 键旋转 |
|---|---|---|---|---|
| 2520~2521 | 5 | 5 | ||
| 2521~2522 | -2 | -2 | ||
| 2523~2524 | -1 | -1 | ||
| 2524~2525 | 2 | 2 | ||
| 2526~2527 | -2 | -2 | ||
| 2527~2528 | 2 | 2 | ||
| 2528~2529 | 1 | 1 | ||
| 2529~2530 | -1 | -1 | ||
| 2530~2531 | 1 | 2 | -1 | |
| 2531~2532 | 0 | |||
| 2532~2533 | 1 | 1 | ||
| 2534~2535 | -1 | -2 | 1 | |
| 2535~2536 | 3 | 2 | 1 | |
| 2536~2537 | -2 | -2 | ||
| 2537~2538 | 1 | |||
| 2538~2539 | 1 | 2 | ||
| 2539~2540 | -1 | -1 | ||
| 2540~2541 | 3 | 3 | ||
| 总计 | 10 | 6 | 1 | 3 |
| Time/ps | δN6 | 缺口收缩/扩大 | 碳链 | 键旋转 |
|---|---|---|---|---|
| 2520~2521 | 5 | 5 | ||
| 2521~2522 | -2 | -2 | ||
| 2523~2524 | -1 | -1 | ||
| 2524~2525 | 2 | 2 | ||
| 2526~2527 | -2 | -2 | ||
| 2527~2528 | 2 | 2 | ||
| 2528~2529 | 1 | 1 | ||
| 2529~2530 | -1 | -1 | ||
| 2530~2531 | 1 | 2 | -1 | |
| 2531~2532 | 0 | |||
| 2532~2533 | 1 | 1 | ||
| 2534~2535 | -1 | -2 | 1 | |
| 2535~2536 | 3 | 2 | 1 | |
| 2536~2537 | -2 | -2 | ||
| 2537~2538 | 1 | |||
| 2538~2539 | 1 | 2 | ||
| 2539~2540 | -1 | -1 | ||
| 2540~2541 | 3 | 3 | ||
| 总计 | 10 | 6 | 1 | 3 |
| [1] |
Xie, S.-Y.; Yang, S.-F.; Li, S.-H. Fullerenes: Fundamental and Application,Science Press, Beijing, 2019. (in Chinese)
|
|
(谢素原, 杨上峰, 李姝慧, 富勒烯: 从基础到应用, 科学出版社, 北京, 2019.)
|
|
| [2] |
Gan, L.-H.; Wang, C.-R. Structure, Properties and Applications of Fullerenes and Their Derivatives, Chemical Industry Press, Beijing, 2019. (in Chinese)
|
|
(甘利华, 王春儒, 富勒烯及其衍生物的结构、性质和应用, 化学工业出版社, 北京, 2019.)
|
|
| [3] |
Qiu, L.; Liang, J.-Y.; Zhang, Z.-X.; Wang, T.-S. Acta Chim. Sinica 2022, 80, 874. (in Chinese)
doi: 10.6023/A22020087 |
|
(邱玲, 梁家艺, 张竹霞, 王太山, 化学学报, 2022, 80, 874.)
doi: 10.6023/A22020087 |
|
| [4] |
Wu, B.; Wang, C.; Li, B.-L.; Wang, C.-R. Acta Chim. Sinica 2022, 80, 101. (in Chinese)
doi: 10.6023/A21120564 |
|
(吴波, 王冲, 李宝林, 王春儒, 化学学报, 2022, 80, 101.)
doi: 10.6023/A21120564 |
|
| [5] |
Ramazani, A.; Moghaddasi, M. A.; Malekzadeh, A. M.; Rezayati, S.; Hanifehpour, Y.; Joo, S. W. Inorg. Chem. Commun. 2021, 125, 108442.
doi: 10.1016/j.inoche.2021.108442 |
| [6] |
Xue, X.-G.; Meng, L.-Y.; Ma, Y.; Zhang, C.-Y. J. Phys. Chem. C 2017, 121, 7502.
doi: 10.1021/acs.jpcc.7b00294 |
| [7] |
Xu, H. M.S. Thesis, Southwest University, Chongqing, 2020. (in Chinese)
|
|
(徐惠, 硕士论文, 西南大学, 重庆, 2020.)
|
|
| [8] |
Howard, J. B.; McKinnon, J. T.; Johnson, M. E.; Makarovsky, Y.; Lafleur, A. L. J. Phys. Chem. 1992, 96, 6657.
doi: 10.1021/j100195a026 |
| [9] |
Homann, K. H. Angew. Chem., Int. Ed. 1998, 37, 2434.
doi: 10.1002/(ISSN)1521-3773 |
| [10] |
Takehara, H.; Fujiwara, M.; Arikawa, M.; Diener, M. D.; Alford, J. M. Carbon 2005, 43, 311.
doi: 10.1016/j.carbon.2004.09.017 |
| [11] |
Zhu, Y.; Zhang, G.; Zhang, W.; Lin, T.; Xie, H.; Liu, Q.; Zhang, H. J. Wuhan Univ. Technol. (Mater. Sci. Ed.) 2007, 22, 94.
|
| [12] |
Sharma, A.; Mukut, K. M.; Roy, S. P.; Goudeli, E. Carbon 2021, 180, 215.
doi: 10.1016/j.carbon.2021.04.065 |
| [13] |
Wang, Y.; Gu, M.-Y.; Wu, J.-J.; Cao, L.; Lin, Y.-Y.; Huang, X. Y. Int. J. Hydrogen Energy 2021, 46, 36557.
doi: 10.1016/j.ijhydene.2021.08.125 |
| [14] |
Zhao, J.; Lin, Y.-Y.; Huang, K.; Gu, M.-Y.; Lu, K.; Chen, P.; Wang, Y.; Zhu, B.-C. Fuel 2020, 262, 116677.
doi: 10.1016/j.fuel.2019.116677 |
| [15] |
Han, S.; Li, X.; Nie, F.; Zheng, M.; Liu, X.; Guo, L. Energy Fuels 2017, 31, 8434.
doi: 10.1021/acs.energyfuels.7b01194 |
| [16] |
Liu, Y.; Wei, X.; Sun, W.-Z.; Zhao, L. Energy Fuels 2021, 35, 16778.
doi: 10.1021/acs.energyfuels.1c02462 |
| [17] |
Zhang, C.-Y.; Zhang, C.; Ma, Y.; Xue, X.-G. Phys. Chem. Chem. Phys. 2015, 17, 11469.
doi: 10.1039/C5CP00926J |
| [18] |
Zhong, R.; Hong, R. Chem. Eng. J. 2020, 387, 124102.
doi: 10.1016/j.cej.2020.124102 |
| [19] |
Li, H. B.; Page, A. J.; Irle, S.; Morokuma, K. J. Phys. Chem. Lett. 2013, 4, 2323.
doi: 10.1021/jz400925f |
| [20] |
Van Duin, A. C.; Dasgupta, S.; Lorant, F.; Goddard, W. A. J. Phys. Chem. A 2001, 105, 9396.
doi: 10.1021/jp004368u |
| [21] |
Brenner, D. W. Phys. Rev. B 1990, 42, 9458.
doi: 10.1103/PhysRevB.42.9458 |
| [22] |
Mao, Q.; Van Duin, A. C.; Luo, K. H. Carbon 2017, 121, 380.
doi: 10.1016/j.carbon.2017.06.009 |
| [23] |
Yoon, K.; Rahnamoun, A.; Swett, J. L.; Iberi, V.; Cullen, D. A.; Vlassiouk, I. V.; Belianinov, A.; Jesse, S.; Sang, X.; Ovchinnikova, O. S.; Rondinone, A. J.; Unocic, R. R.; Van Duin, A. C. ACS Nano. 2016, 10, 8376.
doi: 10.1021/acsnano.6b03036 |
| [24] |
Chen, J.; Pei, J.; Zhao, H. J. Phys. Chem. C 2021, 125, 19345.
doi: 10.1021/acs.jpcc.1c02610 |
| [25] |
Mei, H.; Cui, J.; He, X.; Lu, Y.; Sun, X.; Xu, K.; Mei, X. J. Phys. Chem. C 2022, 126, 13388.
doi: 10.1021/acs.jpcc.2c02784 |
| [26] |
Gaikwad, P. S.; Kowalik, M.; Jensen, B. D.; Van Duin, A.; Odegard, G. M. ACS Appl. Nano Mater. 2022, 5, 5915.
doi: 10.1021/acsanm.2c01280 |
| [27] |
Qian, H. J.; van Duin, A. C.; Morokuma, K.; Irle, S. J. Chem. Theory Comput. 2011, 7, 2040.
doi: 10.1021/ct200197v |
| [28] |
Thompson, A. P.; Aktulga, H. M.; Berger, R.; Bolintineanu, D. S.; Brown, W. M.; Crozier, P. S.; in't Veld, P. J.; Kohlmeyer, A.; Moore, S. G.; Nguyen, T. D.; Shan, R.; Stevens, M. J.; Tranchida, J.; Trott, C.; Plimpton, S. J. Comp. Phys. Commun. 2022, 271, 108171.
doi: 10.1016/j.cpc.2021.108171 |
| [29] |
Plimpton, S. J. Comput. Phys. 1995, 117, 1.
|
| [30] |
Hoover, W. G. Phys. Rev. A 1985, 31, 1695.
doi: 10.1103/PhysRevA.31.1695 |
| [31] |
Humphrey, W.; Dalke, A.; Schulten, K. J. Mol. Graphics 1996, 14, 33.
doi: 10.1016/0263-7855(96)00018-5 |
| [32] |
Qian, H. J.; Wang, Y.; Morokuma, K. Carbon 2017, 114, 635.
doi: 10.1016/j.carbon.2016.12.062 |
| [33] |
Ma, J.; Chen, X.; Song, M.; Wang, C.; Xia, W. Diamond Relat. Mater. 2021, 117, 108445.
doi: 10.1016/j.diamond.2021.108445 |
| [34] |
Saha, B.; Irle, S.; Morokuma, K. J. Phys. Chem. C 2011, 115, 22707.
doi: 10.1021/jp203614e |
| [1] | 王海朋, 蔡文生, 邵学广. 抗冻剂抗冻机制的近红外光谱与分子模拟研究★[J]. 化学学报, 2023, 81(9): 1167-1174. |
| [2] | 韩逸之, 蓝建慧, 刘学, 石伟群. 基于机器学习势函数的熔盐体系分子动力学研究进展[J]. 化学学报, 2023, 81(11): 1663-1672. |
| [3] | 赵珂, 程夏宇, 马雪雪, 耿明慧. 含哌嗪基团锌离子探针的双光子吸收增强机理[J]. 化学学报, 2023, 81(10): 1371-1378. |
| [4] | 眭玉光, 周锦荣, 廖攀, 梁文杰, 徐海. 巨型给-受体分子开关化合物的合成及性质研究[J]. 化学学报, 2022, 80(8): 1061-1065. |
| [5] | 邱玲, 梁家艺, 张竹霞, 王太山. 15N同位素标记的金属氮化物内嵌富勒烯的合成与表征[J]. 化学学报, 2022, 80(7): 874-878. |
| [6] | 王丹, 封波, 张晓昕, 刘亚楠, 裴燕, 乔明华, 宗保宁. 基于热解ZIF-8的氮掺杂碳电化学氧还原合成过氧化氢催化剂[J]. 化学学报, 2022, 80(6): 772-780. |
| [7] | 林文源, 朱清哲, 马云龙, 王鹏, 万硕, 郑庆东. 理性调控聚合物给体-非富勒烯受体的混溶性制备高效率有机太阳能电池※[J]. 化学学报, 2022, 80(6): 724-733. |
| [8] | 吴波, 王冲, 李宝林, 王春儒. 光驱动的金属富勒烯分子磁开关※[J]. 化学学报, 2022, 80(2): 101-104. |
| [9] | 杜英喆, 张恒, 苑世领. Al2O3/PDMS复合材料热传导的分子动力学模拟[J]. 化学学报, 2021, 79(6): 787-793. |
| [10] | 刘长安, 洪士博, 李蓓. 石墨烯在甘油/尿素剥离液中的稳定行为的分子动力学模拟研究[J]. 化学学报, 2021, 79(4): 530-538. |
| [11] | 梁尊, 张鑫, 吕松泰, 梁洪涛, 杨洋. 单分子层受限冰-水共存界线毛细波与固-液相变动力学[J]. 化学学报, 2021, 79(1): 108-118. |
| [12] | 范勤, 梁洪涛, 许贤祺, 吕松泰, 梁尊, 杨洋. 应用极化水分子模型研究单分子层受限水的介电性质[J]. 化学学报, 2020, 78(6): 547-556. |
| [13] | 高斯萌, 夏坤, 康志红, 乃永宁, 袁瑞霞, 牛瑞霞. “拟双子”阴离子表面活性剂在癸烷/水界面的分子动力学模拟[J]. 化学学报, 2020, 78(2): 155-160. |
| [14] | 胡瑜辉, 武文林, 于立扬, 骆开均, 徐小鹏, 李瑛, 彭强. 基于吡咯并吡咯二酮核心的苝二酰亚胺类受体分子的合成及光伏性能[J]. 化学学报, 2020, 78(11): 1246-1254. |
| [15] | 杨鹏里, 王振兴, 梁尊, 梁洪涛, 杨洋. 电场作用下水表面电势的分子动力学研究[J]. 化学学报, 2019, 77(10): 1045-1053. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
