化学学报 ›› 2019, Vol. 77 ›› Issue (9): 856-860.DOI: 10.6023/A19070252 上一篇 下一篇
所属专题: 有机自由基化学
研究通讯
投稿日期:2019-07-04
发布日期:2019-08-15
通讯作者:
刘国生
E-mail:gliu@mail.sioc.ac.cn
基金资助:
Cheng, Zhongming, Chen, Pinhong, Liu, Guosheng*( )
)
Received:2019-07-04
Published:2019-08-15
Contact:
Liu, Guosheng
E-mail:gliu@mail.sioc.ac.cn
Supported by:文章分享
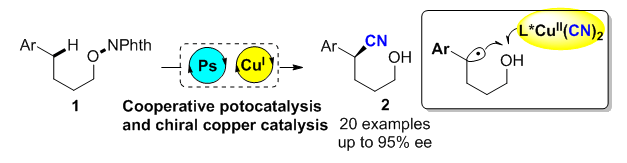
以N-烷氧基邻苯二甲酰亚胺为底物, 利用光与铜共催化, 在蓝光照射下, 实现了远程C—H键不对称氰基化反应. 反应中涉及氧自由基的1,5-氢迁移过程, 结合铜催化自由基接力的策略, 以良好到优秀的对映选择性、单一的区域选择性得到了芳基取代的烷基醇类衍生物δ-位C—H键不对称氰基化产物. 该方法条件温和, 具有较好的官能团兼容性以及底物普适性, 为光学活性的δ-氰基醇类化合物的合成提供了高效方法.
成忠明, 陈品红, 刘国生. 光/铜共催化远程C—H键的不对称氰基化反应[J]. 化学学报, 2019, 77(9): 856-860.
Cheng, Zhongming, Chen, Pinhong, Liu, Guosheng. Enantioselective Cyanation of Remote C—H Bonds via Cooperative Photoredox and Copper Catalysis[J]. Acta Chimica Sinica, 2019, 77(9): 856-860.
| Entry | Photo cat. | Solvent | 2a+2a' Yield (ee)b | 3a+4a Yieldb |
|---|---|---|---|---|
| 1 | Ir(bpy)3 | DMF | 31% (77%) | 47% |
| 2 | [Ir]-1 | DMF | 16% (77%) | 16% |
| 3 | [Ir]-2 | DMF | 30% (77%) | 32% |
| 4 | [Ru] | DMF | 8% (76%) | 11% |
| 5 | Eosin Y | DMF | 0 | 0 |
| 6 | Ir(bpy)3 | DCM | 93% (85%) | 7% |
| 7 | Ir(bpy)3 | PhCF3 | 57% (85%) | 6% |
| 8 | Ir(bpy)3 | CH3CN | 71% (79%) | 27% |
| 9 | Ir(bpy)3 | DCM | 0 | 0 |
| 10 | Ir(bpy)3 | DCM | 0 | 0 |
| 11 | — | DCM | 0 | 0 |
| Entry | Photo cat. | Solvent | 2a+2a' Yield (ee)b | 3a+4a Yieldb |
|---|---|---|---|---|
| 1 | Ir(bpy)3 | DMF | 31% (77%) | 47% |
| 2 | [Ir]-1 | DMF | 16% (77%) | 16% |
| 3 | [Ir]-2 | DMF | 30% (77%) | 32% |
| 4 | [Ru] | DMF | 8% (76%) | 11% |
| 5 | Eosin Y | DMF | 0 | 0 |
| 6 | Ir(bpy)3 | DCM | 93% (85%) | 7% |
| 7 | Ir(bpy)3 | PhCF3 | 57% (85%) | 6% |
| 8 | Ir(bpy)3 | CH3CN | 71% (79%) | 27% |
| 9 | Ir(bpy)3 | DCM | 0 | 0 |
| 10 | Ir(bpy)3 | DCM | 0 | 0 |
| 11 | — | DCM | 0 | 0 |
| [1] |
(a) Wang, J.; Liu, H. Chin. J. Org. Chem. 2012, 32, 1643.
doi: 10.6023/cjoc1202132 |
|
( 王江, 柳红 , 有机化学, 2012, 32, 1643)
doi: 10.6023/cjoc1202132 |
|
|
(b) Fleming, F. F.; Yao, L.; Ravikumar, P. C.; Funk, L.; Shook, B. C. J. Med. Chem. 2010, 53, 7902.
doi: 10.6023/cjoc1202132 |
|
| [2] | (a) Rappoport, Z. The Chemistry of the Cyano Group, Interscience Publishers, London, 1970. |
| (b) Larock, R. C. Comprehensive Organic Transformations: A Guide to Functional Group Preparation, 2nd ed., Wiley-VCH, Weinheim, 1999, p. 821. | |
| [3] |
Cernak, T.; Dykstra, K. D.; Tyagarajan, S.; Vachal, P.; Krska, S. W. Chem. Soc. Rev. 2016, 45, 546.
doi: 10.1039/C5CS00628G |
| [4] |
(a) Meunier, B.; de Visser, S. P.; Shaik, S . Chem. Rev. 2004, 104, 3947.
doi: 10.1021/cr020443g |
|
(b) Ortiz de Montellano, P. R. Chem. Rev. 2010, 110, 932.
doi: 10.1021/cr020443g |
|
| [5] |
For some reviews, see: (a) Che, C. -M.; Lo, V. K.-Y.; Zhou, C.-Y.; Huang, J.-S. Chem. Soc. Rev. 2011, 40, 1950.
doi: 10.1039/c0cs00142b |
|
(b) Lu, H.; Zhang, X. P. Chem. Soc. Rev. 2011, 40, 1899.
doi: 10.1039/c0cs00142b |
|
|
(c) Huang, X.; Groves, J. T. Chem. Rev. 2018, 118, 2491.
doi: 10.1039/c0cs00142b |
|
|
(d) Bietti, M . Angew. Chem., Int. Ed.. 2018, 57, 16618.
doi: 10.1039/c0cs00142b |
|
|
(e) Pei, P.; Zhang, F.; Yi, H.; Lei, A . Acta Chim. Sinica. 2017, 75, 15.
doi: 10.1039/c0cs00142b |
|
|
(裴朋昆, 张凡, 易红, 雷爱文, 化学学报, 2017, 75, 15.
doi: 10.1039/c0cs00142b |
|
| [6] |
For some reviews, see: Stateman, L. M.; Nakafuku, K. M.; Nagib, D. A. Synthesis 2018, 50, 1569.
doi: 10.1055/s-0036-1591930 |
| [7] |
(a) Martínez, C.; Muñiz, K . Angew. Chem., Int. Ed.. 2015, 54, 8287.
doi: 10.1002/anie.201501122 |
|
(b) Choi, G. J.; Zhu, Q.; Miller, D. C.; Gu, C. J.; Knowles, R. R . Nature. 2016, 539, 268.
doi: 10.1002/anie.201501122 |
|
|
(c) Chu, J. C. K.; Rovis, T . Nature. 2016, 539, 272.
doi: 10.1002/anie.201501122 |
|
|
(d) Chen, D.; Chu, J. C. K.; Rovis, T . J. Am. Chem. Soc.. 2017, 139, 14897.
doi: 10.1002/anie.201501122 |
|
|
(e) Wappes, E. A.; Fosu, S. C.; Chopko, T. C.; Nagib, D. A . Angew. Chem., Int. Ed.. 2016, 55, 9974.
doi: 10.1002/anie.201501122 |
|
|
(f) Liu, T.; Myers, M. C.; Yu, J.-Q. . Angew. Chem., Int. Ed.. 2017, 56, 306.
doi: 10.1002/anie.201501122 |
|
|
(g) Becker, P.; Duhamel, T.; Stein, C. J.; Reiher, M.; Muñiz, K . Angew. Chem., Int. Ed.. 2017, 56, 8004.
doi: 10.1002/anie.201501122 |
|
|
(h) Li, Z.; Wang, Q.; Zhu, J . Angew. Chem., Int. Ed.. 2018, 57, 13288.
doi: 10.1002/anie.201501122 |
|
|
(i) Jiang, H.; Studer, A . Angew. Chem., Int. Ed.. 2018, 57, 1692.
doi: 10.1002/anie.201501122 |
|
|
(j) Xia, Y.; Wang, L.; Studer, A . Angew. Chem., Int. Ed.. 2018, 57, 12940.
doi: 10.1002/anie.201501122 |
|
|
(k) Dauncey, E. M.; Morcillo, S. P.; Douglas, J. J.; Sheikh, N. S.; Leonori, D . Angew. Chem., Int. Ed.. 2018, 57, 744.
doi: 10.1002/anie.201501122 |
|
|
(l) Morcillo, S. P.; Dauncey, E. M.; Kim, J. H.; Douglas, J. J.; Sheikh, N. S.; Leonori, D . Angew. Chem., Int. Ed.. 2018, 57, 12945.
doi: 10.1002/anie.201501122 |
|
|
(m) Li, C.; Lang, K.; Lu, H.; Hu, Y.; Cui, X.; Wojtas, L.; Zhang, X. P . Angew. Chem., Int. Ed. 2018, 57, 16837.
doi: 10.1002/anie.201501122 |
|
|
(n) Chen, H.; Guo, L.; Yu, S . Org. Lett.. 2018, 20, 6255.
doi: 10.1002/anie.201501122 |
|
|
(o) Stateman, L. M.; Wappes, E. A.; Nakafuku, K. M.; Edwards, K. M.; Nagib, D. A . Chem. Sci.. 2019, 10, 2693.
doi: 10.1002/anie.201501122 |
|
|
(p) Zhang, Z.; Stateman, L. M.; Nagib, D. A . Chem. Sci.. 2019, 10, 1207.
doi: 10.1002/anie.201501122 |
|
|
(q) Wu, K.; Wang, L.; Colón-Rodríguez, S.; Flechsig, G.-U.; Wang, T . Angew. Chem., Int. Ed.. 2019, 58, 1774.
doi: 10.1002/anie.201501122 |
|
|
(r) Bao, X.; Wang, Q.; Zhu, J . Nature Commun.. 2019, 10, 768.
doi: 10.1002/anie.201501122 |
|
|
(s) Lang, K.; Torker, S.; Wojtas, L.; Zhang, X. P . J. Am. Chem. Soc.. 2019, DOI: 10.1021/jacs.9b05850.
doi: 10.1002/anie.201501122 |
|
| [8] |
(a) Peng, Y.; Lin, J.-S.; Li, L.; Zheng, S.-C.; Xiong, Y.-P.; Zhao, L.-J.; Tan, B.; Liu, X.-Y. Angew. Chem., Int. Ed. 2014, 53, 11890.
doi: 10.1002/anie.201405401 |
|
(b) Zhang, J.; Li, Y.; Zhang, F.; Hu, C.; Chen, Y . Angew. Chem., Int. Ed. 2016, 55, 1872.
doi: 10.1002/anie.201405401 |
|
|
(c) Wang, C. Y.; Harms, K.; Meggers, E . Angew. Chem., Int. Ed.. 2016, 55, 13495.
doi: 10.1002/anie.201405401 |
|
|
(d) Hu, A.; Guo, J.-J.; Pan, H.; Tang, H.; Gao, Z.; Zuo, Z . J. Am. Chem. Soc.. 2018, 140, 1612.
doi: 10.1002/anie.201405401 |
|
|
(e) Zhu, Y.; Huang, K.; Pan, J.; Qiu, X.; Luo, X.; Qin, Q.; Wei, J.; Wen, X.; Zhang, L.; Jiao, N . Nat. Commun.. 2018, 9, 2625.
doi: 10.1002/anie.201405401 |
|
|
(f) Wu, X.; Zhang, H.; Tang, N.; Wu, Z.; Wang, D.; Ji, M.; Xu, Y.; Wang, M.; Zhu, C . Nat. Commun.. 2018, 9, 3343.
doi: 10.1002/anie.201405401 |
|
|
(g) Wu, X.; Wang, M.; Huan, L.; Wang, Wang, D. J.; Zhu, C . Angew. Chem., Int. Ed.. 2018, 57, 1640.
doi: 10.1002/anie.201405401 |
|
|
(h) Wang, M.; Huang, L.; Zhu, C . Org. Lett.. 2019, 21, 821.
doi: 10.1002/anie.201405401 |
|
|
(i) Kim, I.; Park, B.; Kang, G.; Kim, J.; Jung, H.; Lee, H.; Baik, M.; Hong, S . Angew. Chem., Int. Ed.. 2018, 57, 15517.
doi: 10.1002/anie.201405401 |
|
|
(j) Guan, H.; Sun, S.; Mao, Y.; Chen, L.; Lu, R.; Huang, J.; Liu, L . Angew. Chem. Int. Ed.. 2018, 57, 11413.
doi: 10.1002/anie.201405401 |
|
|
(k) Bao, X.; Wang, Q.; Zhu, J . Angew. Chem. Int. Ed.. 2019, 58, 2139.
doi: 10.1002/anie.201405401 |
|
| [9] |
(a) Yu, P.; Zheng, S.-C.; Yang, N.-Y.; Tan, B.; Liu, X.-Y . Angew. Chem., Int. Ed.. 2015, 54, 4041.
doi: 10.1002/anie.201412310 |
|
(b) Cui, X.; Xu, X.; Jin, L.-M.; Wojtasa, L.; Zhang, X. P. Chem. Sci. 2015, 6, 1219.
doi: 10.1002/anie.201412310 |
|
|
(c) Chen, J.-Q.; Wei, Y.-L.; Xu, G.-Q.; Liang, Y.-M.; Xu, P.-F . Chem. Commun.. 2016, 52, 6455.
doi: 10.1002/anie.201412310 |
|
|
(d) Li, T.; Yu, P.; Lin, J.-S.; Zhi, Y.; Liu, X.-Y . Chin. J. Chem.. 2016, 34, 490.
doi: 10.1002/anie.201412310 |
|
|
(e) Li, L.; Ye, L.; Ni, S.-F.; Li, Z.-L.; Chen, S.; Du, Y.-M.; Li, X.-H.; Dang, L.; Liu, X.-Y . Org. Chem. Front.. 2017, 4, 2139.
doi: 10.1002/anie.201412310 |
|
|
(f) Yuan, W.; Zhou. Z.; Gong, L.; Meggers, E . Chem. Commun.. 2017, 53, 8964.
doi: 10.1002/anie.201412310 |
|
|
(g) Li, T.; Yu, P.; Du, Y.-M.; Lin, J.-S.; Zhi, Y.; Liu, X.-Y . J. Fluorine Chem. 2017, 203, 210.
doi: 10.1002/anie.201412310 |
|
|
(h) Wang, N.; Ye, L.; Li, Z.-L.; Li, L.; Li, Z.; Zhang, H.-X.; Guo, Z.; Gu, Q.-S.; Liu, X.-Y . Org. Chem. Front. 2018, 5, 2810.
doi: 10.1002/anie.201412310 |
|
|
(i) Chen, J.-Q.; Chang, R.; Lin, J.-B.; Luo, Y.-C.; Xu, P.-F . Org. Lett. 2018, 20, 2395.
doi: 10.1002/anie.201412310 |
|
|
(j) Wang, Y.; Wen, X.; Cui, X.; Zhang, X. P . J. Am. Chem. Soc. 2018, 140, 4792.
doi: 10.1002/anie.201412310 |
|
|
(k) Wen, X.; Wang, Y.; Zhang, X. P . Chem. Sci. 2018, 9, 5082.
doi: 10.1002/anie.201412310 |
|
|
(l) Wu, S.; Wu, X.; Wang, D.; Zhu, C . Angew. Chem., Int. Ed.. 2019, 58, 1499.
doi: 10.1002/anie.201412310 |
|
|
(m) Chuentragool, P.; Yadagiri, D.; Morita, T.; Sarkar, S.; Parasram, M.; Wang, Y.; Gevorgyan, V . Angew. Chem. Int. Ed.. 2019, 58, 1794.
doi: 10.1002/anie.201412310 |
|
| [10] |
Wang, F.; Chen, P.; Liu, G . Acc. Chem. Res. 2018, 51, 2036.
doi: 10.1021/acs.accounts.8b00265 |
| [11] |
(a) Zhang, W.; Wang, F.; McCann, S. D.; Wang, D.; Chen, P.; Stahl, S. S.; Liu, G . Science 2016, 353, 1014.
doi: 10.1126/science.aaf7783 |
|
(b) Zhang, W.; Wu, L.; Chen, P.; Liu, G . Angew. Chem., Int. Ed. 2019, 58, 6425.
doi: 10.1126/science.aaf7783 |
|
|
(c) Zhang, W.; Chen, P.; Liu, G. J. Am. Chem. Soc. 2017, 139, 7709.
doi: 10.1126/science.aaf7783 |
|
| [12] |
For cyanations see: (a) Wang, F.; Wang, D.; Wan, X.; Wu, L.; Chen, P.; Liu, G. J. Am. Chem. Soc. 2016, 138, 15547.
doi: 10.1021/jacs.6b10468 |
|
(b) Wang, D.; Wang, F.; Chen, P.; Lin, Z.; Liu, G. Angew. Chem., Int. Ed. 2017, 56, 2054.
doi: 10.1021/jacs.6b10468 |
|
|
(c) Lu, F.-D.; Liu, D.; Zhu, L.; Lu, L.-Q.; Yang, Q.; Zhou, Q.-Q.; Wei, Y.; Lan, Y.; Xiao, W.-J . J. Am. Chem. Soc. 2019, 141, 6167.
doi: 10.1021/jacs.6b10468 |
|
|
For arylations, see:(d) Wu, L.; Wang, F.; Wan, X.; Wang, D.; Chen, P.; Liu, G . J. Am. Chem. Soc.. 2017, 139, 2904.
doi: 10.1021/jacs.6b10468 |
|
|
(e) Wang, D.; Wu, L.; Wang, F.; Wan, X.; Chen, P.; Lin, Z.; Liu, G . J. Am. Chem. Soc.. 2017, 139, 6811.
doi: 10.1021/jacs.6b10468 |
|
|
For alkynylation, see: (f) Fu, L.; Zhou, S.; Wan, X.; Chen, P.; Liu, P . J. Am. Chem. Soc.. 2018, 140, 10965.
doi: 10.1021/jacs.6b10468 |
|
| [13] |
Wang, D.; Zhu, N.; Chen, P.; Lin, Z.; Liu, G . J. Am. Chem. Soc. 2017, 139, 15632.
doi: 10.1021/jacs.7b09802 |
| [14] |
(a) Curran, D. P.; Kim, D.; Liu, H. T.; Shen, W . J. Am. Chem. Soc.. 1988, 110, 5900.
doi: 10.1021/ja00225a052 |
|
(b) Kim, S.; Lee, T. A.; Song, Y . Synlett 1998, 471. (c) Zlotorzynska, M.; Sammis, G. M. Org. Lett. 2011, 13, 6264.
doi: 10.1021/ja00225a052 |
| [1] | 陈健强, 朱钢国, 吴劼. 镍催化氮杂环丙烷的开环偶联反应研究[J]. 化学学报, 2024, 82(2): 190-212. |
| [2] | 吴宇晗, 张栋栋, 尹宏宇, 陈正男, 赵文, 匙玉华. “双碳”目标下Janus In2S2X光催化还原CO2的密度泛函理论研究[J]. 化学学报, 2023, 81(9): 1148-1156. |
| [3] | 鱼章龙, 李忠良, 杨昌江, 顾强帅, 刘心元. 铜催化的二醇类化合物对映选择性去对称化反应研究进展★[J]. 化学学报, 2023, 81(8): 955-966. |
| [4] | 何明慧, 叶子秋, 林桂庆, 尹晟, 黄心翊, 周旭, 尹颖, 桂波, 汪成. 卟啉基共价有机框架的光催化研究进展★[J]. 化学学报, 2023, 81(7): 784-792. |
| [5] | 刘嘉文, 林玮璜, 王惟嘉, 郭学益, 杨英. Cu1.94S-SnS纳米异质结的合成及其光催化降解研究[J]. 化学学报, 2023, 81(7): 725-734. |
| [6] | 刘坜, 郑刚, 范国强, 杜洪光, 谭嘉靖. 4-酰基/氨基羰基/烷氧羰基取代汉斯酯参与的有机反应研究进展[J]. 化学学报, 2023, 81(6): 657-668. |
| [7] | 李飞, 丁汇丽, 李超忠. 基于氟仿衍生的三氟甲基硼络合物参与的烯烃氢三氟甲基化反应[J]. 化学学报, 2023, 81(6): 577-581. |
| [8] | 徐袁利, 潘辉, 杨义, 左智伟. 连续流条件下蒽-铈协同催化的苄位碳氢键选择性氧化反应★[J]. 化学学报, 2023, 81(5): 435-440. |
| [9] | 黄家翩, 刘飞, 吴劼. 二氟环丙烯参与的有机反应研究进展★[J]. 化学学报, 2023, 81(5): 520-532. |
| [10] | 齐学平, 王飞, 张健. 后合成法构筑钛基金属有机框架及其应用[J]. 化学学报, 2023, 81(5): 548-558. |
| [11] | 陈健强, 朱钢国, 吴劼. 草酸酯类化合物在自由基脱羟基化反应中的研究进展[J]. 化学学报, 2023, 81(11): 1609-1623. |
| [12] | 孟庆端, 韩佳宏, 潘一骁, 郝伟, 范青华. C1-对称手性氮杂环卡宾(NHC)配体的不对称合成及其催化性能研究★[J]. 化学学报, 2023, 81(10): 1271-1279. |
| [13] | 邱孔茜, 李杰, 马浩文, 周伟, 蔡倩. 捕捉环加成反应中的有机亚铜中间体构筑氮杂环化合物研究进展[J]. 化学学报, 2023, 81(1): 42-63. |
| [14] | 杨春晖, 陈景超, 李新汉, 孟丽, 王凯民, 孙蔚青, 樊保敏. 可见光催化的硅烷二氟烯丙基化反应[J]. 化学学报, 2023, 81(1): 1-5. |
| [15] | 解众舒, 薛中鑫, 许子文, 李倩, 王洪宇, 李维实. 石墨相氮化碳的共轭交联修饰及其对可见光催化产氢性能的影响[J]. 化学学报, 2022, 80(9): 1231-1237. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||