化学学报 ›› 2021, Vol. 79 ›› Issue (9): 1107-1112.DOI: 10.6023/A21070320 上一篇 下一篇
研究通讯
投稿日期:2021-07-11
发布日期:2021-08-17
通讯作者:
游书力
基金资助:
Qing-Ru Zhaoa,b, Ru Jianga, Shu-Li Youa,b( )
)
Received:2021-07-11
Published:2021-08-17
Contact:
Shu-Li You
Supported by:文章分享
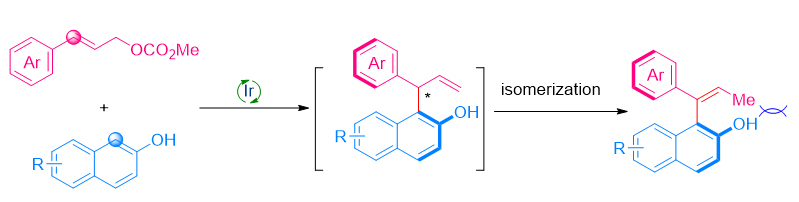
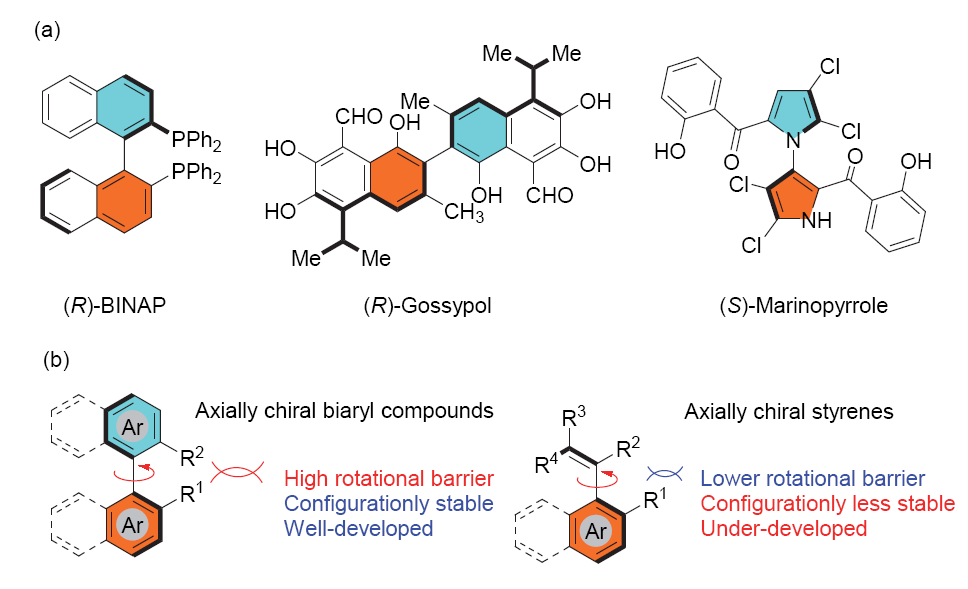
轴手性化合物是一类重要的手性化合物, 其中苯乙烯类轴手性化合物因其轴手性稳定性相对较差, 目前高效不对称合成的方法比较局限. 本工作以β-萘酚作为亲核试剂, 通过将金属铱催化不对称烯丙基取代与双键异构化串联, 实现了中心手性到轴手性的转移, 从而高效地合成了一系列β-萘酚衍生的苯乙烯类轴手性化合物.
赵庆如, 蒋茹, 游书力. 铱催化串联不对称烯丙基取代/双键异构化构建轴手性化合物[J]. 化学学报, 2021, 79(9): 1107-1112.
Qing-Ru Zhao, Ru Jiang, Shu-Li You. Ir-catalyzed Sequential Asymmetric Allylic Substitution/Olefin Isomerization for the Synthesis of Axially Chiral Compounds[J]. Acta Chimica Sinica, 2021, 79(9): 1107-1112.
| Entry | Base | Solvent | L | T/℃ | 4aa | |||
|---|---|---|---|---|---|---|---|---|
| Yieldb | eec | |||||||
| 1 | DBU | THF | L1 | 25 | 68% | 77% | ||
| 2 | DABCO | THF | L1 | 25 | 90% | 87% | ||
| 3 | TBD | THF | L1 | 25 | 17% | 49% | ||
| 4 | Cs2CO3 | THF | L1 | 25 | 83% | 43% | ||
| 5 | t-BuONa | THF | L1 | 25 | 48% | 83% | ||
| 6 | DABCO | 1,4-dioxane | L1 | 25 | 59% | 71% | ||
| 7 | DABCO | Et2O | L1 | 25 | 91% | 93% | ||
| 8 | DABCO | DCM | L1 | 25 | 36% | 74% | ||
| 9 | DABCO | toluene | L1 | 25 | 98% | 72% | ||
| 10 | DABCO | MeCN | L1 | 25 | 38% | 55% | ||
| 11 | DABCO | Et2O | L2 | 25 | 96% | 90% | ||
| 12 | DABCO | Et2O | L3 | 25 | trace | N.D.d | ||
| 13 | DABCO | Et2O | L4 | 25 | 12% | 55% | ||
| 14 | DABCO | Et2O | L5 | 25 | trace | N.D.d | ||
| 15 | DABCO | Et2O | L1 | 20 | 97% (93%e) | 94% | ||
| 16 | DABCO | Et2O | L1 | 10 | 82% | 95% | ||
| 17f | DABCO | Et2O | L1 | 20 | 84% | 90% | ||
| 18g | DABCO | Et2O | L1 | 20 | 72% | 84% | ||
| Entry | Base | Solvent | L | T/℃ | 4aa | |||
|---|---|---|---|---|---|---|---|---|
| Yieldb | eec | |||||||
| 1 | DBU | THF | L1 | 25 | 68% | 77% | ||
| 2 | DABCO | THF | L1 | 25 | 90% | 87% | ||
| 3 | TBD | THF | L1 | 25 | 17% | 49% | ||
| 4 | Cs2CO3 | THF | L1 | 25 | 83% | 43% | ||
| 5 | t-BuONa | THF | L1 | 25 | 48% | 83% | ||
| 6 | DABCO | 1,4-dioxane | L1 | 25 | 59% | 71% | ||
| 7 | DABCO | Et2O | L1 | 25 | 91% | 93% | ||
| 8 | DABCO | DCM | L1 | 25 | 36% | 74% | ||
| 9 | DABCO | toluene | L1 | 25 | 98% | 72% | ||
| 10 | DABCO | MeCN | L1 | 25 | 38% | 55% | ||
| 11 | DABCO | Et2O | L2 | 25 | 96% | 90% | ||
| 12 | DABCO | Et2O | L3 | 25 | trace | N.D.d | ||
| 13 | DABCO | Et2O | L4 | 25 | 12% | 55% | ||
| 14 | DABCO | Et2O | L5 | 25 | trace | N.D.d | ||
| 15 | DABCO | Et2O | L1 | 20 | 97% (93%e) | 94% | ||
| 16 | DABCO | Et2O | L1 | 10 | 82% | 95% | ||
| 17f | DABCO | Et2O | L1 | 20 | 84% | 90% | ||
| 18g | DABCO | Et2O | L1 | 20 | 72% | 84% | ||
| Entry | Condition | NMR yield of 4aa | ee of 3aa | ee of 4aa |
|---|---|---|---|---|
| 1 | [Ir(cod)Cl]2 (2 mol%) (S,S,Sa)-L1 (4 mol%) DBU (3.0 equiv.) | 68% | 80% | 77% |
| 2 | Without DBU | 8% | 94% | 80% |
| 3 | Without [Ir] catalyst | 7% | 80% | 71% |
| Entry | Condition | NMR yield of 4aa | ee of 3aa | ee of 4aa |
|---|---|---|---|---|
| 1 | [Ir(cod)Cl]2 (2 mol%) (S,S,Sa)-L1 (4 mol%) DBU (3.0 equiv.) | 68% | 80% | 77% |
| 2 | Without DBU | 8% | 94% | 80% |
| 3 | Without [Ir] catalyst | 7% | 80% | 71% |
| [1] |
For reviews: (a) Kumarasamy, E.; Raghunathan, R.. Sibi, M. P.. Sivaguru, J. Chem. Rev. 2015, 115, 11239.
doi: 10.1021/acs.chemrev.5b00136 pmid: 26414162 |
|
(b) Wang, Y.-B.; Tan, B. Acc. Chem. Res. 2018, 51, 534.
doi: 10.1021/acs.accounts.7b00602 pmid: 26414162 |
|
|
(c) Zhang, S.; Liao, G.; Shi, B. Chin. J. Org. Chem. 2019, 39, 1522. (in Chinese)
doi: 10.6023/cjoc201904030 pmid: 26414162 |
|
|
( 张硕, 廖港, 史炳锋, 有机化学, 2019, 39, 1522.)
doi: 10.6023/cjoc201904030 pmid: 26414162 |
|
|
(d) Cheng, J. K.; Xiang, S.-H.; Li, S.; Ye, L.; Tan, B. Chem. Rev. 2021, 121, 4805.
doi: 10.1021/acs.chemrev.0c01306 pmid: 26414162 |
|
| [2] |
For reviews: (a) Kozlowski, M. C.; Morgan, B. J.; Linton, E. C. Chem. Soc. Rev. 2009, 38, 3193.
doi: 10.1039/b821092f pmid: 26346838 |
|
(b) Bringmann, G.; Gulder, T.; Gulder, T. A.; Breuning, M. Chem. Rev. 2011, 111, 563.
doi: 10.1021/cr100155e pmid: 26346838 |
|
|
(c) Erbas-Cakmak, S.; Leigh, D. A.; McTernan, C. T.; Nussbaumer, A. L. Chem. Rev. 2015, 115, 10081.
doi: 10.1021/acs.chemrev.5b00146 pmid: 26346838 |
|
| [3] |
Miyashita, A.; Yasuda, A.; Takaya, H.; Toriumi, K.; Ito, T.; Souchi, T.; Noyori, R. J. Am. Chem. Soc. 1980, 102, 7932.
doi: 10.1021/ja00547a020 |
| [4] |
For books: (a) Zhou, Q.-L. Privileged Chiral Ligands and Catalysts, Wiley-VCH, Weinheim, Germany, 2011.
|
|
(b) You, S.-L. Asymmetric Dearomatization Reactions, Wiley-VCH, Weinheim, Germany, 2016. For review:
|
|
|
(c) Li, Y.-M.; Kwong, F.-Y.; Yu, W.-Y.; Chan, A. S. C. Coord. Chem. Rev. 2007, 251, 2119.
doi: 10.1016/j.ccr.2007.07.020 |
|
| [5] |
For reviews: (a) Wencel-Delord, J.; Panossian, A.; Leroux, F. R.; Colobert, F. Chem. Soc. Rev. 2015, 44, 3418.
doi: 10.1039/c5cs00012b pmid: 25904287 |
|
(b) Loxq, P.; Manoury, E.; Poli, R.; Deydier, E.; Labande, A. Coord. Chem. Rev. 2016, 308, 131.
doi: 10.1016/j.ccr.2015.07.006 pmid: 25904287 |
|
|
(c) Wang, Q.; Gu, Q.; You, S.-L. Acta Chim. Sinica 2019, 77, 690. (in Chinese)
doi: 10.6023/A19060222 pmid: 25904287 |
|
|
( 王强, 顾庆, 游书力, 化学学报, 2019, 77, 690.)
doi: 10.6023/A19060222 pmid: 25904287 |
|
| [6] |
Gu, Z.; Feng, J. SynOpen 2021, 5, 68.
doi: 10.1055/s-0040-1706028 |
| [7] |
For selected examples: (a) Zheng, S.-C.; Wu, S.; Zhou, Q.; Chung, L. W.; Ye, L.; Tan, B. Nat. Commun. 2017, 8, 15238.
doi: 10.1038/ncomms15238 pmid: 30463406 |
|
(b) Tan, Y.; Jia, S.; Hu, F.; Liu, Y.; Peng, L.; Li, D.; Yan, H. J. Am. Chem. Soc. 2018, 140, 16893.
doi: 10.1021/jacs.8b09893 pmid: 30463406 |
|
|
(c) Jia, S.; Chen, Z.; Zhang, N.; Tan, Y.; Liu, Y.; Deng, J.; Yan, H. J. Am. Chem. Soc. 2018, 140, 7056.
doi: 10.1021/jacs.8b03211 pmid: 30463406 |
|
|
(d) Wang, C.-S.; Li, T.-Z.; Liu, S.-J.; Zhang, Y.-C.; Deng, S.; Jiao, Y.; Shi, F. Chin. J. Chem. 2020, 38, 543.
doi: 10.1002/cjoc.v38.6 pmid: 30463406 |
|
|
(e) Sheng, F.-T.; Li, Z.-M.; Zhang, Y.-Z.; Sun, L.-X.; Zhang, Y.-C.; Tan, W.; Shi, F. Chin. J. Chem. 2020, 38, 583.
doi: 10.1002/cjoc.v38.6 pmid: 30463406 |
|
|
(f) Wang, J.-Y.; Sun, M.; Yu, X.-Y.; Zhang, Y.-C.; Tan, W.; Shi, F. Chin. J. Chem. 2021, 39, 2163.
doi: 10.1002/cjoc.v39.8 pmid: 30463406 |
|
| [8] |
For selected examples: (a) Bringmann, G.; Price Mortimer, A. J.; Keller, P. A.; Gresser, M. J.; Garner, J.; Breuning, M.. Angew. Chem. Int. Ed. 2005, 44, 5384.
doi: 10.1002/(ISSN)1521-3773 |
|
(b) Pan, C.; Zhu, Z.; Zhang, M.; Gu, Z. Angew. Chem. Int. Ed. 2017, 56, 4777.
doi: 10.1002/anie.201701467 |
|
| [9] |
For selected examples: (a) Wang, F.; Qi, Z.; Zhai, S.; Zheng, G.; Mi, R.; Huang, Z.; Zhu, X.; He, X.; Li, X. Angew. Chem. Int. Ed. 2020, 59, 13288.
doi: 10.1002/anie.v59.32 pmid: 34163646 |
|
(b) Yang, C.; Wu, T.-R.; Li, Y.; Wu, B.-B.; Jin, R.-X.; Hu, D.-D.; Li, Y.-B.; Bian, K.-J.; Wang, X.-S. Chem. Sci. 2021, 12, 3726.
doi: 10.1039/d0sc06661c pmid: 34163646 |
|
| [10] |
Feng, J.; Li, B.; He, Y.; Gu, Z. Angew. Chem. Int. Ed. 2016, 55, 2186.
doi: 10.1002/anie.201509571 |
| [11] |
Sun, C.; Qi, X.; Min, X.-L.; Bai, X.-D.; Liu, P.; He, Y. Chem. Sci. 2020, 11, 10119.
doi: 10.1039/D0SC02828B |
| [12] |
For reviews of allylic substitution reactions: (a) Zhang, M.-M.; Luo, Y.-Y.; Lu, L.-Q.; Xiao, W.-J. Acta Chim. Sinica 2018, 76, 838. (in Chinese)
doi: 10.6023/A18060237 |
|
( 张毛毛, 骆元元, 陆良秋, 肖文精, 化学学报, 2018, 76, 838.)
doi: 10.6023/A18060237 |
|
|
(b) Ma, X.; Yu, J.; Wang, Z.; Zhang, Y.; Zhou, Q. Chin. J. Org. Chem. 2020, 40, 2669. (in Chinese)
doi: 10.6023/cjoc202005013 |
|
|
马献涛, 于静, 王子龙, 张赟, 周秋菊, 有机化学, 2020, 40, 2669.)
|
|
|
For selected examples of allylic substitution reactions: (c) Yao, K.; Liu, H.; Yuan, Q.; Liu, Y.; Liu, D.; Zhang, W. Acta Chim. Sinica 2019, 77, 993. (in Chinese)
doi: 10.6023/A19060210 |
|
|
( 姚坤, 刘浩, 袁乾家, 刘燕刚, 刘德龙, 张万斌, 化学学报, 2019, 77, 993.)
doi: 10.6023/A19060210 |
|
|
(d) Xiao, J.; Xu, H.; Huo, X.; Zhang, W.; Ma, S. Chin. J. Chem. 2021, 39, 1958.
doi: 10.1002/cjoc.v39.7 |
|
|
(e) Huo, X.; Zhao, L.; Luo, Y.; Wu, Y.; Sun, Y.; Li, G.; Gridneva, T.; Zhang, J.; Ye, Y.; Zhang, W. CCS Chem. 2021, 3, 1933.
|
|
| [13] |
For reviews of iridium-catalyzed allylic substitution reactions: (a) Hartwig, J. F.; Stanley, L. M.. Acc. Chem. Res. 2010, 43, 1461.
doi: 10.1021/ar100047x |
|
(b) Qu, J.; Helmchen, G. Acc. Chem. Res. 2017, 50, 2539.
doi: 10.1021/acs.accounts.7b00300 |
|
|
(c) Deng, Y.; Yang, W.; Yang, X.; Yang, D. Chin. J. Org. Chem. 2017, 37, 3039. (in Chinese)
doi: 10.6023/cjoc201704034 |
|
|
( 邓颖颍, 杨文, 杨新, 杨定乔, 有机化学, 2017, 37, 3039.)
doi: 10.6023/cjoc201704034 |
|
|
(d) Cheng, Q.; Tu, H.-F.; Zheng, C.; Qu, J.-P.; Helmchen, G.; You, S.-L. Chem. Rev. 2019, 119, 1855.
doi: 10.1021/acs.chemrev.8b00506 |
|
|
(e) Tian, F.; Zhang, J.; Yang, W.; Deng, W. Chin. J. Org. Chem. 2020, 40, 3262. (in Chinese)
doi: 10.6023/cjoc202005008 |
|
|
田飞, 张键, 杨武林, 邓卫平, 有机化学, 2020, 40, 3262.
doi: 10.6023/cjoc202005008 |
|
| [14] |
For selected examples of iridium-catalyzed allylic substitution reactions: (a) Krautwald, S.; Sarlah, D.; Schafroth, M. A.; Carreira, E. M. Science 2013, 340, 1065.
doi: 10.1126/science.1237068 pmid: 23723229 |
|
(b) Liu, W.-B.; Reeves, C. M.; Stoltz, B. M. J. Am. Chem. Soc. 2013, 135, 17298.
doi: 10.1021/ja4097829 pmid: 23723229 |
|
|
(c) Liu, J.; Cao, C.-G.; Sun, H.-B.; Zhang, X.; Niu, D. J. Am. Chem. Soc. 2016, 138, 13103.
doi: 10.1021/jacs.6b05288 pmid: 23723229 |
|
|
(d) Huo, X.; He, R.; Zhang, X.; Zhang, W. J. Am. Chem. Soc. 2016, 138, 11093.
doi: 10.1021/jacs.6b06156 pmid: 23723229 |
|
|
(e) Huo, X.; Zhang, J.; Fu, J.; He, R.; Zhang, W. J. Am. Chem. Soc. 2018, 140, 2080.
doi: 10.1021/jacs.8b00187 pmid: 23723229 |
|
|
(f) Wei, L.; Zhu, Q.; Xu, S.-M.; Chang, X.; Wang, C.-J. J. Am. Chem. Soc. 2018, 140, 1508.
doi: 10.1021/jacs.7b12174 pmid: 23723229 |
|
|
(g) Xu, S.-M.; Wei, L.; Shen, C.; Xiao, L.; Tao, H.-Y.; Wang, C.-J. Nat. Commun. 2019, 10, 5553.
doi: 10.1038/s41467-019-13529-z pmid: 23723229 |
|
|
(h) Han, M.; Yang, M.; Wu, R.; Li, Y.; Jia, T.; Gao, Y.; Ni, H.-L.; Hu, P.; Wang, B.-Q.; Cao, P. J. Am. Chem. Soc. 2020, 142, 13398.
doi: 10.1021/jacs.0c01766 pmid: 23723229 |
|
|
(i) Yang, P.; Liu, C.-X.; Zhang, W.-W.; You, S.-L. Acta Chim. Sinica. 2021, 79, 742. (in Chinese)
doi: 10.6023/A21050198 pmid: 23723229 |
|
|
( 杨普苏, 刘晨旭, 张文文, 游书力, 化学学报, 2021, 79, 742.)
doi: 10.6023/A21050198 pmid: 23723229 |
|
| [15] |
For selected examples: (a) Liu, W.-B.; He, H.; Dai, L.-X.; You, S.-L. Org. Lett. 2008, 10, 1815.
doi: 10.1021/ol800409d pmid: 33479149 |
|
(b) Wu, Q.-F.; He, H.; Liu, W.-B.; You, S.-L. J. Am. Chem. Soc. 2010, 132, 11418.
doi: 10.1021/ja105111n pmid: 33479149 |
|
|
(c) Huang, L.; Dai, L.-X.; You, S.-L. J. Am. Chem. Soc. 2016, 138, 5793.
doi: 10.1021/jacs.6b02678 pmid: 33479149 |
|
|
(d) Jiang, S.-Z.; Zeng, X.-Y.; Liang, X.; Lei, T.; Wei, K.; Yang, Y.-R. Angew. Chem. Int. Ed. 2016, 55, 4044.
doi: 10.1002/anie.201511549 pmid: 33479149 |
|
|
(e) Tu, H.-F.; Zhang, X.; Zheng, C.; Zhu, M.; You, S.-L. Nat. Catal. 2018, 1, 601.
doi: 10.1038/s41929-018-0111-8 pmid: 33479149 |
|
|
(f) Huang, L.; Cai, Y.; Zhang, H.-J.; Dai, L.-X.; You, S.-L. CCS Chem. 2019, 1, 106.
pmid: 33479149 |
|
|
(g) Uno, H.; Kawai, K.; Shiro, M.; Shibata, N. ACS Catal. 2020, 10, 14117.
doi: 10.1021/acscatal.0c03927 pmid: 33479149 |
|
|
(h) Jiang, R.; Ding, L.; Zheng, C.; You, S.-L. Science 2021, 371, 380.
doi: 10.1126/science.abd6095 pmid: 33479149 |
|
|
(i) Zhang, J.; Gao, Y.-S.; Gu, B.-M.; Yang, W.-L.; Tian, B.-X.; Deng, W.-P. ACS Catal. 2021, 11, 3810.
doi: 10.1021/acscatal.1c00081 pmid: 33479149 |
|
| [16] |
For selected examples: (a) Zhuo, C.-X.; Liu, W.-B.; Wu, Q.-F.; You, S.-L. Chem. Sci. 2012, 3, 205.
doi: 10.1039/C1SC00517K |
|
(b) Zhuo, C.-X.; Wu, Q.-F; Zhao, Q.; Xu, Q.-L.; You, S.-L. J. Am. Chem. Soc. 2013, 135, 8169.
doi: 10.1021/ja403535a |
|
|
(c) Zhuo, C.-X.; Cheng, Q.; Liu, W.-B.; Zhao, Q.; You, S.-L. Angew. Chem. Int. Ed. 2015, 54, 8475.
doi: 10.1002/anie.201502259 |
|
|
(d) Huang, L.; Cai, Y.; Zheng, C.; Dai, L.-X.; You, S.-L. Angew. Chem. Int. Ed. 2017, 56, 10545.
doi: 10.1002/anie.v56.35 |
|
|
(e) Zi, Y.; Lange, M.; Schultz, C.; Vilotijevic, I. Angew. Chem. Int. Ed. 2019, 58, 10727.
doi: 10.1002/anie.v58.31 |
|
| [17] |
For selected examples: (a) Bechem, B.; Patman, R. L.; Hashmi, A. S. K.; Krische, M. J. J. Org. Chem. 2010, 75, 1795.
doi: 10.1021/jo902697g |
|
(b) Chen, W.; Hartwig, J. F. J. Am. Chem. Soc. 2012, 134, 15249.
doi: 10.1021/ja306850b |
|
| [18] |
For selected examples: (a) Nemoto, T.; Ishige, Y.; Yoshida, M.; Kohno, Y.; Kanematsu, M.; Hamada, Y. Org. Lett. 2010, 12, 5020.
doi: 10.1021/ol102190s |
|
(b) Wu, Q.-F.; Liu, W.-B.; Zhuo, C.-X.; Rong, Z.-Q.; Ye, K.-Y.; You, S.-L. Angew. Chem., Int. Ed. 2011, 50, 4455.
doi: 10.1002/anie.201100206 |
|
|
(c) Xu, Q.-L.; Dai, L.-X.; You, S.-L. Org. Lett. 2012, 14, 2579.
doi: 10.1021/ol3008793 |
|
|
(d) Zhuo, C.-X.; You, S.-L. Angew. Chem., Int. Ed. 2013, 52, 10056.
doi: 10.1002/anie.201304591 |
|
|
(e) Cheng, Q.; Wang, Y.; You, S.-L. Angew. Chem., Int. Ed. 2016, 55, 3496.
doi: 10.1002/anie.201511519 |
|
|
(f) Tu, H.-F.; Zheng, C.; Xu, R.-Q.; Liu, X.-J.; You, S.-L. Angew. Chem. Int. Ed. 2017, 56, 3237.
doi: 10.1002/anie.201609654 |
|
|
(g) Shen, D.; Chen, Q.; Yan, P.; Zeng, X.; Zhong, G. Angew. Chem. Int. Ed., 2017, 56, 3242.
doi: 10.1002/anie.201609693 |
|
| [19] |
Computational studies of the isomerization process were developed by He and coworkers: Wang, J.; Qi, X.; Min, X.-L.; Yi, W.; Liu, P.; He, Y. J. Am. Chem. Soc. 2021, 143, 10686.
doi: 10.1021/jacs.1c04400 |
| [20] |
For selected examples: (a) Bartels, B.; García-Yebra, C.; Helmchen, G. Eur. J. Org. Chem. 2003, 1097.
|
|
(b) Alexakis, A.; Polet, D. Org. Lett. 2004, 6, 3529.
doi: 10.1021/ol048607y |
|
|
(c) Tissot-Croset, K.; Polet, D.; Alexakis, A. Angew. Chem. Int. Ed. 2004, 43, 2426.
doi: 10.1002/(ISSN)1521-3773 |
|
|
(d) Spiess, S.; Welter, C.; Franck, G.; Taquet, J.-P.; Helmchen, G. Angew. Chem. Int. Ed. 2008, 47, 7652.
doi: 10.1002/anie.v47:40 |
|
|
(e) Spiess, S.; Raskatov, J. A.; Gnamm, C.; Brodner, K.; Helmchen, G. Chem. Eur. J. 2009, 15, 11087.
doi: 10.1002/(ISSN)1521-3765 |
|
|
(f) Raskatov, J. A.; Spiess, S.; Gnamm, C.; Brodner, K.; Rominger, F.; Helmchen, G. Chem. Eur. J. 2010, 16, 6601.
doi: 10.1002/chem.200903465 |
|
| [21] |
For selected examples: (a) de Vries, A. H. M.; Meetsma, A.; Feringa, B. L. Angew. Chem. Int. Ed. 1996, 35, 2374.
doi: 10.1002/(ISSN)1521-3773 pmid: 19432473 |
|
(b) Feringa, B. L.; Pineschi, M.; Arnold, L. A.; Imbos, R.; de Vries, A. H. M. Angew. Chem. Int. Ed. 1997, 36, 2620.
doi: 10.1002/(ISSN)1521-3773 pmid: 19432473 |
|
|
(c) Ohmura, T.; Hartwig, J. F. J. Am. Chem. Soc. 2002, 124, 15164.
doi: 10.1021/ja028614m pmid: 19432473 |
|
|
(d) López, F.; Ohmura, T.; Hartwig, J. F. J. Am. Chem. Soc. 2003, 125, 3426.
doi: 10.1021/ja029790y pmid: 19432473 |
|
|
(e) Kiener, C. A.; Shu, C.; Incarvito, C.; Hartwig, J. F. J. Am. Chem. Soc. 2003, 125, 14272.
doi: 10.1021/ja038319h pmid: 19432473 |
|
|
(f) Madrahimov, S. T.; Markovic, D.; Hartwig, J. F. J. Am. Chem. Soc. 2009, 131, 7228.
doi: 10.1021/ja902609g pmid: 19432473 |
|
| [22] |
Leitner, A.; Shekhar, S.; Pouy, M. J.; Hartwig, J. F. J. Am. Chem. Soc. 2005, 127, 15506.
doi: 10.1021/ja054331t |
| [1] | 鱼章龙, 李忠良, 杨昌江, 顾强帅, 刘心元. 铜催化的二醇类化合物对映选择性去对称化反应研究进展★[J]. 化学学报, 2023, 81(8): 955-966. |
| [2] | 杨爽, 王宁宜, 杭青青, 张宇辰, 石枫. 邻羟基苯基取代的对亚甲基苯醌参与的催化不对称反应研究进展★[J]. 化学学报, 2023, 81(7): 793-808. |
| [3] | 王瑞祥, 赵庆如, 顾庆, 游书力. 金/铱接力催化炔基酰胺环化/不对称烯丙基苄基化串联反应★[J]. 化学学报, 2023, 81(5): 431-434. |
| [4] | 高杨, 张学鑫, 余金生, 周剑. α-手性三级叠氮化合物的不对称催化合成新进展★[J]. 化学学报, 2023, 81(11): 1590-1608. |
| [5] | 何倩, 李杰, 喻思佳, 吴东坪, 叶剑良, 黄培强. 铱催化叔酰胺与呋喃硅醚间的类插烯Aldol缩合反应: γ-亚苄基-丁烯酸内酯的合成★[J]. 化学学报, 2023, 81(10): 1265-1270. |
| [6] | 孟庆端, 韩佳宏, 潘一骁, 郝伟, 范青华. C1-对称手性氮杂环卡宾(NHC)配体的不对称合成及其催化性能研究★[J]. 化学学报, 2023, 81(10): 1271-1279. |
| [7] | 张崃, 肖检, 王雅雯, 彭羽. 苯酚(醌)与烯烃的不对称[3+2]环化反应: 手性二氢苯并呋喃的合成进展[J]. 化学学报, 2022, 80(8): 1152-1164. |
| [8] | 马进越, 黄露霏, 周宝文, 姚琳. 无机手性纳米结构的可控构筑及其催化研究进展[J]. 化学学报, 2022, 80(11): 1507-1523. |
| [9] | 杨普苏, 刘晨旭, 张文文, 游书力. 铱催化中氮茚衍生物的Friedel-Crafts类型不对称烯丙基取代反应[J]. 化学学报, 2021, 79(6): 742-746. |
| [10] | 穆博帅, 张志豪, 武文彪, 余金生, 周剑. 手性1,2-二氢吡啶化合物的合成研究进展[J]. 化学学报, 2021, 79(6): 685-693. |
| [11] | 杨妲, 张龙力, 刘欢, 杨朝合. 双功能配体修饰的Ir催化剂在“氢甲酰化-缩醛化”串联反应中的共催化作用[J]. 化学学报, 2021, 79(5): 658-662. |
| [12] | 李翼, 徐明华. 不对称Petasis反应在手性胺类化合物合成中的应用[J]. 化学学报, 2021, 79(11): 1345-1359. |
| [13] | 朱仁义, 廖奎, 余金生, 周剑. P-手性膦氧化物的不对称催化合成研究进展[J]. 化学学报, 2020, 78(3): 193-216. |
| [14] | 张洪浩, 俞寿云. 过渡金属与光氧化还原协同催化的烯丙基取代反应的研究进展[J]. 化学学报, 2019, 77(9): 832-840. |
| [15] | 王强, 顾庆, 游书力. 过渡金属催化不对称C—H键官能团化反应构建轴手性联芳基化合物研究进展[J]. 化学学报, 2019, 77(8): 690-704. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||