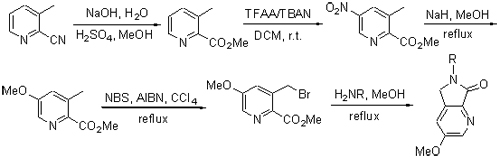
| [1] D'Amato, R. J.; Loughnan, M. S.; Flynn, E.; Folkman, J. Proc. Natl. Acad. Sci. U. S. A. 1994, 91, 4082. [2] Dunzendorfer, S.; Schratzberger, P.; Reinisch, N.; Kahler, C. M.; Wiedermann, C. J. Naunyn. Schmiedeberg's Arch. Pharmacol. 1997, 356, 529. [3] Richardson, P.; Hideshima, T.; Anderson, K. Annu. Rev. Med. 2002, 53, 629. [4] Chen, L. H.; Li, D. D. Chin. J. New Drugs 2006, 15, 1141 (in Chinese).(陈立慧, 李电东, 中国新药杂志, 2006, 15, 1141.) [5] Greig, N. H.; Holloway, H.; Brossi, A.; Zhu, X. X.; Giordano, T.; Yu, Q. S.; Figg, W. D. WO 2005028436, 2005 [Chem. Abstr. 2005, 142, 355170].[6] Miyachi, H.; Azuma, A.; Ogasawara, A.; Uchimura, E.; Watanabe, N.; Kobayashi, Y.; Kato, F.; Kato, M.; Hashimoto, Y. J. Med. Chem. 1997, 40, 2858. [7] Man, H. W.; Corral, L. G.; Stirling, D. I.; Muller, G. W. Bioorg. Med. Chem. Lett. 2003, 13, 3415. [8] Kotla, V.; Goel, S.; Nischal, S.; Heuck, C.; Vivek, K.; Das, B.; Verma, A. J. Hematol. Oncol. 2009, 2, 36. [9] Kaplan, G.; Sampaio, E. P. US 5385901, 1992 [Chem. Abstr. 1992, 117, 226313].[10] Kennenth, S. B.; Shannon, C. D.; William, D. F. Biochem. Pharmacol. 1998, 55, 1827. [11] Fan, B.-L. Ph.D. Dissertation, Shanghai Jiaotong University, Shanghai, 2008 (in Chinese).(范柏林, 博士论文, 上海交通大学, 上海, 2008.) [12] Shi, Q.-F. M.S. Thesis, Shanghai Jiaotong University, Shanghai, 2012 (in Chinese).(史群峰, 硕士论文, 上海交通大学, 上海, 2012.) [13] Kale, R.; Tayade, P.; Saraf, M.; Juvekar, A. Drug Dev. Ind. Pharm. 2008, 34, 149. [14] Shibata, Y.; Sasaki, K.; Hashimoto, Y.; Iwasaki, S. Chem. Pharm. Bull. 1996, 44, 156. [15] Capitosti, S. M.; Hansen, T. P.; Brown, M. L. Bioorg. Med. Chem. 2004, 12, 327. [16] Zhou, H. Ph.D. Dissertation, Shanghai Jiaotong University, Shanghai, 2013 (in Chinese).(周恒, 博士论文, 上海交通大学, 上海, 2013.) [17] Xie, Y.; Wu, G. Q.; Zheng, S. C.; Chen, F. E. Chin. J. Med. Chem. 2006, 16, 49 (in Chinese).(谢艳, 吾国强, 郑士才, 陈芬儿, 中国药物化学杂志, 2006, 16, 49.) [18] Zhang, W.-J.; Luo, Y.; Zhang, Y. M. Chin. J. Pharm. 2006, 37, 434 (in Chinese).(张万金, 罗艳, 张燕梅, 中国医药工业杂志, 2006, 37, 434.) [19] Njoroge, F. G.; Vibulbhan, B.; Pinto, P.; Chan, T. M.; Osterman, R.; Remiszewski, S.; Rosario, J. D.; Doll, R.; Girijavallabhan, V.; Ganguly, A. K. J. Org. Chem. 1998, 63, 445. [20] Wang, Y.-H.; Song, Y. J.; Hu, Z.; Jing, J. H.; Meng, X. L.; Huang, Y. D. Chin. J. Org. Chem. 2009, 29, 780 (in Chinese).(王艳红, 宋元军, 胡 桢, 景介辉, 孟祥丽, 黄玉东, 有机化学, 2009, 29, 780.) [21] Xie, C.; Runnegar, M. T. C.; Snider, B. B. J. Am. Chem. Soc. 2000, 122, 5017. [22] Clarke, K.; Goulding, J.; Scrowston, R. M. J. Chem. Soc., Perkin Trans. 1 1984, 1501. [23] Zhang, E.; Li, C.; Zhang, B. Y.; Lü, A. Q.; Fang, Y.; Feng, S. Q.; Liu, H. M. Chin. J. Org. Chem. 2013, 33, 1100 (in Chinese).(张恩, 李聪, 张保寅, 吕爱桥, 方园, 冯思琦, 刘宏民, 有机化学, 2013, 33, 1100.) [24] Mamiko, S.; Yohei, M.; Hiroki, K.; Hiroyuki, K.; Aya, T.; Kazuo, N.; Yuichi, H. Chem. Pharm. Bull. 2003, 51, 1098. [25] Caroline, S.; Jean-michel, R. J. Enzyme Inhib. Med. Chem. 2008, 23, 659. [26] Yang, M.; He, J. B.; Cheng, Y. X.; Jiang, S. Chin. J. Org. Chem. 2013, 33, 1319 (in Chinese).(杨梅, 何江波, 程永现, 蒋晟, 有机化学, 2013, 33, 1319.) [27] Du, C.; Ren, Y. J.; Wang, Q. W.; Jin, L. Chin. J. Org. Chem. 2013, 33, 1279 (in Chinese).(杜成, 任玉杰, 王庆伟, 金鹭, 有机化学, 2013, 33, 1279.) |