有机化学 ›› 2021, Vol. 41 ›› Issue (11): 4353-4360.DOI: 10.6023/cjoc202107020 上一篇 下一篇
研究论文
马豪杰a, 孙洲b, 刘金磊a, 张夏b, 崔华莉a, 张玉琦a,*( ), 王记江a
), 王记江a
收稿日期:2021-07-07
修回日期:2021-07-26
发布日期:2021-08-24
通讯作者:
张玉琦
作者简介:基金资助:
Haojie Maa, Zhou Sunb, Jinlei Liua, Xia Zhangb, Huali Cuia, Yuqi Zhanga( ), Jijiang Wanga
), Jijiang Wanga
Received:2021-07-07
Revised:2021-07-26
Published:2021-08-24
Contact:
Yuqi Zhang
About author:Supported by:文章分享
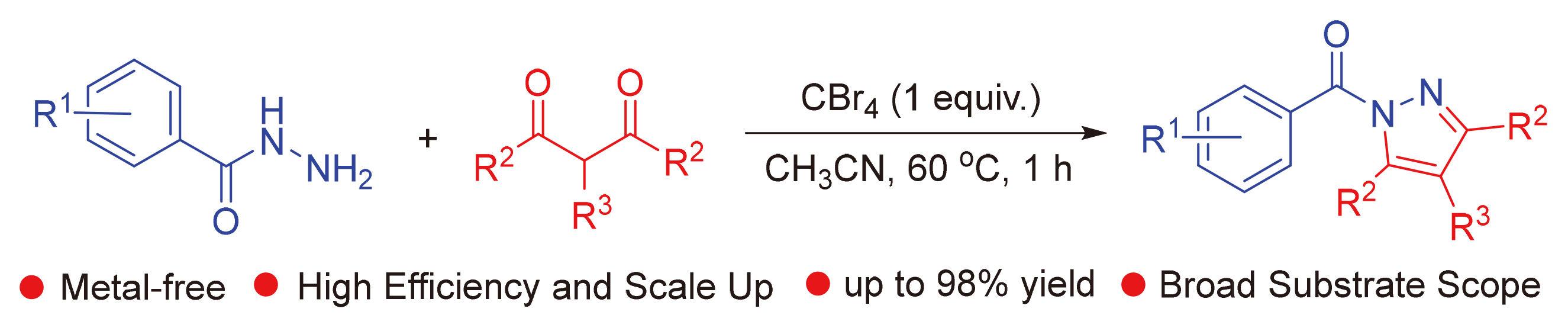
报道了CBr4促进的苯甲酰肼和2,4-戊二酮分子间环化高产率地合成(3,5-二甲基-1H-吡唑-1-基)(苯基)-甲酮. 该方法具有条件温和、良好的官能团耐受性、环境友好、操作简单、成本低、步骤经济、可进行按比例放大等优点, 为广泛应用于药物、生物活性分子和农药中的吡唑类化合物的合成提供了一种实用而有吸引力的策略.
马豪杰, 孙洲, 刘金磊, 张夏, 崔华莉, 张玉琦, 王记江. CBr4促进的分子间环化反应: 高效合成取代N-酰基吡唑[J]. 有机化学, 2021, 41(11): 4353-4360.
Haojie Ma, Zhou Sun, Jinlei Liu, Xia Zhang, Huali Cui, Yuqi Zhang, Jijiang Wang. CBr4-Mediated Intermolecular Cyclization Reaction: Efficient Synthesis of Substituted N-Acylpyrazoles[J]. Chinese Journal of Organic Chemistry, 2021, 41(11): 4353-4360.
| Entry | CBr4 | Solvent | Temp./℃ | Yieldb/% | |
|---|---|---|---|---|---|
| 1 | 1 | DMSO | 60 | 53 | |
| 2 | 1 | DMF | 60 | 85 | |
| 3 | 1 | Toluene | 60 | 15 | |
| 4 | 1 | DCE | 60 | 95 | |
| 5 | 1 | CH3CN | 60 | 98 | |
| 6 | 0.5 | CH3CN | 60 | 76 | |
| 7 | 1.5 | CH3CN | 60 | 93 | |
| 8 | 1 | CH3CN | r.t. | 20 | |
| 9 | 1 | CH3CN | 40 | 96 | |
| 10 | 1 | CH3CN | 80 | 95 | |
| 11c | 1 | CH3CN | 60 | 70 | |
| 12d | 1 | CH3CN | 60 | 95 | |
| Entry | CBr4 | Solvent | Temp./℃ | Yieldb/% | |
|---|---|---|---|---|---|
| 1 | 1 | DMSO | 60 | 53 | |
| 2 | 1 | DMF | 60 | 85 | |
| 3 | 1 | Toluene | 60 | 15 | |
| 4 | 1 | DCE | 60 | 95 | |
| 5 | 1 | CH3CN | 60 | 98 | |
| 6 | 0.5 | CH3CN | 60 | 76 | |
| 7 | 1.5 | CH3CN | 60 | 93 | |
| 8 | 1 | CH3CN | r.t. | 20 | |
| 9 | 1 | CH3CN | 40 | 96 | |
| 10 | 1 | CH3CN | 80 | 95 | |
| 11c | 1 | CH3CN | 60 | 70 | |
| 12d | 1 | CH3CN | 60 | 95 | |
| [1] |
(a) Mantenuto, S.; Ciccolini, C.; Lucarini, S.; Piersanti, G.; Favi, G.; Mantellini, F. Org. Lett. 2017, 19, 608.
doi: 10.1021/acs.orglett.6b03775 pmid: 28094964 |
|
(b) Geng, R.; Zhao, Y.; Li, Y.; Liu, X.; Wang, M. Chin. J. Org. Chem. 2019, 39, 3574. (in Chinese)
doi: 10.6023/cjoc201906017 pmid: 28094964 |
|
|
(耿瑞, 赵宇, 李益豪, 刘鑫磊, 王明安, 有机化学, 2019, 39, 3574.)
doi: 10.6023/cjoc201906017 pmid: 28094964 |
|
|
(c) Ma, H.; Guo, C.; Zhan, Z.; Lu, G.; Zhang, Y. X.; Luo, X.; Cui, X.; Huang, G. New J. Chem. 2017, 41, 5280.
doi: 10.1039/C7NJ01293D pmid: 28094964 |
|
|
(d) Zhong, L.; Jiang, T.; Zhang, F.; Fu, Q.; Liu, X.; Xu, T.; Ding, C.; Chen, J.; Yuan, J.; Tan, C. Chin. J. Org. Chem. 2019, 39, 2655. (in Chinese)
doi: 10.6023/cjoc201903056 pmid: 28094964 |
|
|
(钟良坤, 江涛, 张帆, 付庆, 刘幸海, 许天明, 丁成荣, 陈杰, 袁静, 谭成侠, 有机化学, 2019, 39, 2655.)
pmid: 28094964 |
|
|
(e) Cao, M.; Fang, Y.; Wang, Y.; Xu, X.; Xi, Z.; Tang, S. ACS Comb. Sci. 2020, 22, 268.
doi: 10.1021/acscombsci.0c00012 pmid: 28094964 |
|
|
(f) Gong, C. C.; Tan, H. Y.; Zhang, Q. Chin. J. Org. Chem. 2018, 38, 3086. (in Chinese)
doi: 10.6023/cjoc201805020 pmid: 28094964 |
|
|
(龚超超, 谈寒一, 张倩, 有机化学, 2018, 38, 3086.)
doi: 10.6023/cjoc201805020 pmid: 28094964 |
|
|
(g) Chen, M. M.; Shao, L. Y.; Lun, L. J.; Wu, Y. L.; Fu, X. P.; Ji, Y. F. Chin. Chem. Lett. 2019, 30, 702.
doi: 10.1016/j.cclet.2018.09.022 pmid: 28094964 |
|
| [2] |
(a) He, Y.; Wang, Y. C.; Hu, K.; Xu, X. L.; Wang, H. S.; Pan, Y. M. J. Org. Chem. 2016, 81, 11813.
doi: 10.1021/acs.joc.6b02288 |
|
(b) Murugesan, D.; Mital, A.; Kaiser, M.; Shackleford, D. M.; Morizzi, J.; Katneni, K.; Campbell, M.; Hudson, A.; Charman, S. A.; Yeates, C.; Gilbert, I. H. J. Med. Chem. 2013, 56, 2975.
doi: 10.1021/jm400009c |
|
|
(c) Zhong, Y. Y.; Yu, L. J.; He, Q. Y.; Zhu, Q. Y.; Zhang, C. G.; Cui, X. P.; Zheng, J. X.; Zhao, S. Q. ACS Appl. Mater. Inter. 2019, 11, 32769.
doi: 10.1021/acsami.9b11754 |
|
|
(d) Shi, Y. J.; Zhou, Q.; Wang, Y.; Qian, H. W.; Ye, L. Y.; Feng, X.; Chen, H.; Li, Y. T.; Dai, H.; Wei, Z. H.; Wu, J. M. Chin. J. Org. Chem. 2018, 38, 2450. (in Chinese)
doi: 10.6023/cjoc201806030 |
|
|
(石玉军, 周钱, 王杨, 钱宏炜, 叶林玉, 冯霞, 陈辉, 李雅婷, 戴红, 魏中昊, 吴锦明, 有机化学, 2018, 38, 2450.)
doi: 10.6023/cjoc201806030 |
|
| [3] |
(a) Wang, S. H.; Zhang, B. H.; Chen, J.; Zheng, Y. Y.; Feng, N.; Ma, A. J.; Xu, X. T.; Abdullah, M. A. Chin. J. Org. Chem. 2020, 40, 15. (in Chinese)
doi: 10.6023/cjoc201906007 pmid: 15214796 |
|
(王少华, 张帮红, 陈洁, 郑莹莹, 冯娜, 马爱军, 徐学涛, Abdullah, M. A., 有机化学, 2020, 40, 15.)
doi: 10.6023/cjoc201906007 pmid: 15214796 |
|
|
(b) Li, Y.; Wan, J. P. Chin. J. Org. Chem. 2020, 40, 3889. (in Chinese)
doi: 10.6023/cjoc202005026 pmid: 15214796 |
|
|
(李毅, 万结平, 有机化学, 2020, 40, 3889.)
doi: 10.6023/cjoc202005026 pmid: 15214796 |
|
|
(c) Liu, J. J.; Sun, J.; Fang, Y. B.; Yang, Y. A.; Jiao, R.H.; Zhu, H. L. Org. Biomol. Chem. 2014, 12, 998.
doi: 10.1039/c3ob41953c pmid: 15214796 |
|
|
(d) Ouyang, G.; Cai, X. J.; Chen, Z.; Song, B. A.; Bhadury, P. S.; Yang, S.; Jin, L. H.; Xue, W.; Hu, D. Y.; Zeng, S. J. Agric. Food Chem. 2008, 56, 10160.
doi: 10.1021/jf802489e pmid: 15214796 |
|
|
(e) Tanitame, A.; Oyamada, Y.; Ofuji, K.; Fujimoto, M.; Iwai, N.; Hiyama, Y.; Suzuki, K.; Ito, H.; Terauchi, H.; Kawasaki, M.; Nagai, K.; Wachi, M.; Yamagishi, J. J. Med. Chem. 2004, 47, 3693.
pmid: 15214796 |
|
|
(f) Tian, L. H.; Wan, J. P.; Sheng, Sh. R. ChemCatChem. 2020, 12, 2533.
doi: 10.1002/cctc.v12.9 pmid: 15214796 |
|
| [4] |
(a) Abd El Razik, H. A.; Badr, M. H.; Atta, A. H.; Mouneir, S. M.; Abu-Serie, M. M. Arch. Pharm. 2017, 350, e1700026.
|
|
(b) Bhosle, M. R.; Mali, J. R.; Pal, S.; Srivastava, A. K.; Mane, R. A. Bioorg. Med. Chem. Lett. 2014, 24, 2651.
doi: 10.1016/j.bmcl.2014.04.064 |
|
|
(c) El-Sayed, M. A. A.; Abdel-Aziz, N. I.; Abdel-Aziz, A. A. M.; El-Azab, A. S.; ElTahir, K. E. H. Bioorg. Med. Chem. 2012, 20, 3306.
|
|
|
(d) Bekhit, A. A.; Abdel-Aziem, T. Bioorg. Med. Chem. 2004, 12, 1935.
doi: 10.1016/j.bmc.2004.01.037 |
|
|
(e) Zhang, M.; Zheng, D. D.; Ni, Y. D.; Liang, K.; Xun, X.; Hu, L. P.; Chen, Q. W.; Yang, B.; Liang, Zh. P.; Ding, Y.; Shi, J.; Dai, H. Chin. J. Org. Chem. 2020, 40, 1772. (in Chinese)
doi: 10.6023/cjoc202001028 |
|
|
(张敏, 郑丹丹, 倪亚丹, 梁凯, 荀校, 胡兰萍, 陈庆文, 杨冰, 梁志鹏, 丁颖, 石健, 戴红, 有机化学, 2020, 40, 1772.)
doi: 10.6023/cjoc202001028 |
|
|
(f) Li, Y.; Wan, J. P.; Liu, Y. Y. ChemistrySelect 2020, 5, 9431.
doi: 10.1002/slct.v5.30 |
|
| [5] |
Cox, S. R.; Lesman, S. P.; Boucher, J. F.; Krautmann, M. J.; Hummel, B. D.; Savides, M.; Marsh, S.; Fielde, A.; Stegemann, M. R. J. Vet. Pharmacol. Ther. 2010, 33, 461.
doi: 10.1111/j.1365-2885.2010.01165.x pmid: 20840390 |
| [6] |
Kidwai, M.; Jain, A.; Poddar, R. J. Organomet. Chem. 2011, 696, 1939.
doi: 10.1016/j.jorganchem.2010.09.012 |
| [7] |
Lange, J. H. M.; van Stuivenberg, H. H.; Coolen, H. K. A. C.; Adolfs, T. J. P.; McCreary, A. C.; Keizer, H. G.; Wals, H. C.; Veerman, W.; Borst, A. J. M.; de Looff, W.; Verveer, P. C.; Kruse, C. G. J. Med. Chem. 2005, 48, 1823.
pmid: 15771428 |
| [8] |
(a) Szabo, G.; Fischer, J.; Kis-Varga, A.; Gyires, K. J. Med. Chem. 2008, 51, 142.
doi: 10.1021/jm070821f pmid: 17321742 |
|
(b) Navidpour, L.; Amini, M.; Shafaroodi, H.; Adbi, K.; Ghaheremani, H. M.; Dehpour, R. A.; Shafiee, A. Bioorg. Med. Chem. Lett. 2006, 16, 4483.
pmid: 17321742 |
|
|
(c) Jorand-Lebrun, C.; Brondyk, B.; Jing, L.; Magar, S.; Murray, R.; Reddy, A.; Shroff, H.; Wands, H.; Weiser, Q.; Xu, W.; McKenna, S.; Brugger, N. Bioorg. Med. Chem. Lett. 2007, 17, 2080.
pmid: 17321742 |
|
| [9] |
(a) Zeng, J. L.; Xu, Z. H.; Ma, J. A. Chin. J. Org. Chem. 2020, 40, 1105. (in Chinese)
doi: 10.6023/cjoc201912024 |
|
(曾俊良, 许志红, 马军安, 有机化学, 2020, 40, 1105.)
doi: 10.6023/cjoc201912024 |
|
|
(b) Zhu, Y.; Zheng, D. D.; Miao, H. Y.; Qian, C.; Dai, H.; Liang, K.; Zhou, B. B.; Shi, Y. J.; Xun, X.; Wang, Y. Chin. J. Org. Chem. 2020, 40, 4315. (in Chinese)
doi: 10.6023/cjoc202008031 |
|
|
(朱玥, 郑丹丹, 缪何一, 钱程, 戴红, 梁凯, 周贝贝, 石玉军, 荀校, 王阳, 有机化学, 2020, 40, 4315.)
doi: 10.6023/cjoc202008031 |
|
| [10] |
Chen, X.; She, J.; Shang, Z.; Wu, J.; Wu, H.; Zhang, P. Synthesis 2008, 3478.
|
| [11] |
Polsheettiwar, V.; Varma, S. R. Tetrahedron Lett. 2008, 49, 397.
|
| [12] |
Xiong, W.; Chen, J. X.; Liu, M. C.; Ding, J. C.; Wu, H. Y.; Su, W. K. J. Braz. Chem. Soc. 2009, 20, 367.
doi: 10.1590/S0103-50532009000200023 |
| [13] |
Texier-Boullet, F.; Klein, B.; Hamelin, J. Synth. Commun. 1986, 409.
|
| [14] |
(a) Yang, G. P.; He, X.; Yu, B.; Hu, C. W. Appl. Organomet. Chem. 2018, 32, 4532.
|
|
(b) Wang, H. F.; Sun, X. L.; Zhang, S. L.; Liu, G. L.; Wang, C. J.; Zhu, L. L.; Zhang, H. Synlett 2018, 29, 2689.
doi: 10.1055/s-0037-1610330 |
|
|
(c) Venkateswarlu, V.; Kour, J.; Aravinda Kumar, K. A.; Verma, P. K.; Lakshma Reddy, G.; Hussain, Y.; Tabassum, A.; Balgotra, S.; Gupta, S.; Hudwekar, A. D.; Vishwakarma, R. A.; Sawant, S. D. RSC Adv. 2018, 8, 26523.
doi: 10.1039/C8RA04550J |
|
| [15] |
Curini, M.; Rosati, O.; Campagna, V.; Montanari, F.; Cravatto, G.; Boclini, M. Synlett 2005, 2927.
|
| [16] |
Wang, Z.; Qin, H. Green Chem. 2004, 6, 90.
doi: 10.1039/b312833d |
| [17] |
Polshettiwar, V.; Varma, R. S. Tetrahedron 2010, 66, 1091.
doi: 10.1016/j.tet.2009.11.015 |
| [1] | 王翔, 陈平, 支三军, 胡华友, 阚玉和. 四溴化碳促进下2-(1H-苯并[d]咪唑)-3-芳基丙烯腈的高效合成[J]. 有机化学, 2018, 38(11): 3123-3126. |
| [2] | 张文婷, 孙健, 徐飞, 朱红, 岳瑞雪, 张毅, 钮福祥. 离子液体中碘催化下2-氨基苯甲酰肼和4-氧代庚二酸的反应研究[J]. 有机化学, 2017, 37(12): 3191-3197. |
| [3] | 王树良, 盛洁, 屠树江, 王香善. 水介质中Yb(OTf)3催化下喹唑啉酮衍生物的绿色合成[J]. 有机化学, 2011, 31(9): 1522-1526. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||