有机化学 ›› 2021, Vol. 41 ›› Issue (11): 4347-4352.DOI: 10.6023/cjoc202107031 上一篇 下一篇
所属专题: 热点论文虚拟合集
研究论文
黄利a, 王毓浩a, 刘吉英a, 李世俊a,*( ), 张文静a,*(
), 张文静a,*( ), 蓝宇a,b,*(
), 蓝宇a,b,*( )
)
收稿日期:2021-07-15
修回日期:2021-08-15
发布日期:2021-08-24
通讯作者:
李世俊, 张文静, 蓝宇
基金资助:
Li Huanga, Yuhao Wanga, Jiying Liua, Shijun Lia( ), Wenjing Zhanga(
), Wenjing Zhanga( ), Yu Lana,b(
), Yu Lana,b( )
)
Received:2021-07-15
Revised:2021-08-15
Published:2021-08-24
Contact:
Shijun Li, Wenjing Zhang, Yu Lan
Supported by:文章分享
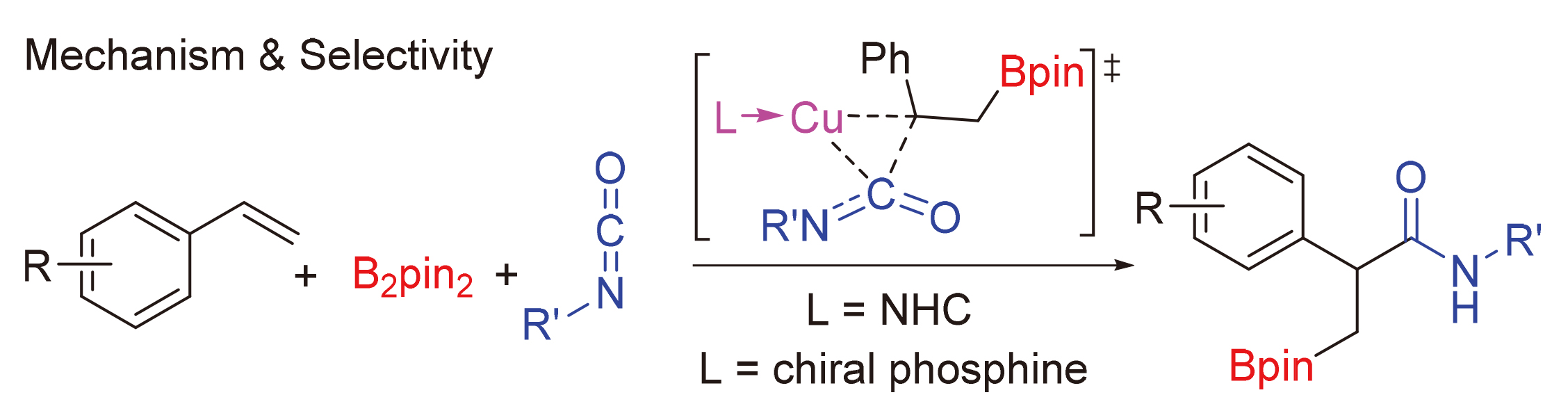
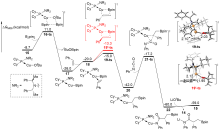
异氰酸酯作为一种重要的单碳碳源, 在合成化学中可以用于制备酰胺衍生物或杂环类化合物, 其活化和转化机制是一个重要的问题. 通过密度泛函理论(DFT)计算研究了亚铜催化异氰酸酯氢硼乙基化反应的机理. 研究结果表明, 该反应中叔丁醇亚铜是活性催化物种. 反应经历了叔丁醇亚铜与硼烷转金属化、烯烃插入、异氰酸酯插入、与叔丁醇锂转金属化再生叔丁醇亚铜等步骤. 其中, 异氰酸酯插入为决速步, 经历了一个特殊的三元环过渡态. 如果使用手性的亚膦酰胺作为配体, 则可实现立体选择性的异氰酸酯氢硼乙基化. 对映体选择性控制步为烯烃插入, 配体的空间调控决定了立体选择性. 反应决速步同样是异氰酸酯插入, 但相对使用卡宾配体, 该过程活化能较低. 因此, 亚膦酰胺-亚铜催化体系可能具有较高催化活性.
黄利, 王毓浩, 刘吉英, 李世俊, 张文静, 蓝宇. 铜催化异氰酸酯加成反应机理研究[J]. 有机化学, 2021, 41(11): 4347-4352.
Li Huang, Yuhao Wang, Jiying Liu, Shijun Li, Wenjing Zhang, Yu Lan. Mechanistic Study of Cu-Catalyzed Addition Reaction of lsocyanates[J]. Chinese Journal of Organic Chemistry, 2021, 41(11): 4347-4352.


| [1] |
Monie, F.; Grignard, B.; Thomassin, J.-M.; Mereau, R.; Tassaing, T.; Jerome, C.; Detrembleur, C. Angew. Chem., nt. Ed. 2020, 59, 17033.
|
| [2] |
Allen, M. A.; Ivanovich, R. A.; Beauchemin, A. M. Angew. Chem., nt. Ed. 2020, 59, 23188.
|
| [3] |
Sharpe, H. R.; Geer, A. M.; Lewis, W.; Blake, A. J.; Kays, D. L. Angew. Chem.,Int. Ed. 2017, 56, 4845.
doi: 10.1002/anie.201701051 |
| [4] |
Zheng, S.; Primer, D. N.; Molander, G. A. ACS Catal. 2017, 7, 7957.
doi: 10.1021/acscatal.7b02795 |
| [5] |
Mohjer, F.; Mofatehnia, P.; Rangraz, Y.; Heravi, M. M. J. Organomet. Chem. 2021, 936, 121712.
doi: 10.1016/j.jorganchem.2021.121712 |
| [6] |
Cui, C.-X.; Chen, H.; Li, S.-J.; Zhang, T.; Qu, L.-B.; Lan, Y. Coord. Chem. Rev. 2020, 412, 213251.
doi: 10.1016/j.ccr.2020.213251 |
| [7] |
Zhang, C.; Zhu, Y. Y.; Wei, D. H.; Sun, D. Z.; Zhang, W. J.; Tang, M. S. J. Phys. Chem. A 2010, 114, 2913.
doi: 10.1021/jp910173d pmid: 20136163 |
| [8] |
Khan, A.; Xing, J. X.; Zhao, J. M.; Kan, Y. H.; Zhang, W. B.; Zhang, Y. J. Chem.-Eur. J. 2015, 21, 120.
doi: 10.1002/chem.201405830 |
| [9] |
Yingcharoen, P.; Natongchai, W.; Poater, A.; D'Elia, V. Catal. Sci. Technol. 2020, 10, 5544.
doi: 10.1039/D0CY00987C |
| [10] |
Bruffaerts, J.; von Wolff, N.; Diskin-Posner, Y.; Ben-David, Y.; Milstein, D. J. Am. Chem. Soc. 2019, 141, 16486.
doi: 10.1021/jacs.9b08942 pmid: 31532664 |
| [11] |
Yang, Y.; Anker, M. D.; Fang, J.; Mahon, M. F.; Maron, L.; Weetman, C.; Hill, M. S. Chem. Sci. 2017, 8, 3529.
doi: 10.1039/c7sc00117g pmid: 30155199 |
| [12] |
Lu, C. R.; Hu, L. J.; Zhao, B.; Yao, Y. M. Organometallics 2019, 38, 2167.
doi: 10.1021/acs.organomet.9b00147 |
| [13] |
Yang, Y.; Canty, A. J.; O'Hair, R. A. J. J. Mass Spectrom. 2021, 56, e4579.
|
| [14] |
Alves, L. G.; Madeira, F.; Munhá, R. F.; Barros, S.; Veiros, L. F.; Martins, A. M. Dalton Trans. 2015, 44, 1441.
doi: 10.1039/c4dt02851a pmid: 25427676 |
| [15] |
Fiorito, D.; Liu, Y.; Besnard, C.; Mazet, C. J. Am. Chem. Soc. 2020, 142, 623.
doi: 10.1021/jacs.9b12297 |
| [16] |
Peris, E. Chem. Rev. 2018, 118, 9988.
doi: 10.1021/acs.chemrev.6b00695 |
| [17] |
Kuwata, S.; Hahn, F. E. Chem. Rev. 2018, 118, 9642.
doi: 10.1021/acs.chemrev.8b00176 |
| [18] |
Xu, G. Q.; Senanayake, C. H.; Tang, W. J. Acc. Chem. Res. 2019, 52, 1101.
doi: 10.1021/acs.accounts.9b00029 |
| [19] |
Fu, W. Z.; Tang, W. J. ACS Catal. 2016, 6, 4814.
doi: 10.1021/acscatal.6b01001 |
| [20] |
de Figueiredo, R. M.; Suppo, J.-S.; Campagne, J.-M. Chem. Rev. 2016, 116, 12029.
pmid: 27673596 |
| [21] |
(a) Kohn, W.; Sham, L. J. Phys. Rev. 1965, 140, A1133.
doi: 10.1103/PhysRev.140.A1133 |
|
(b) Kohn, W. Rev. Mod. Phys. 1999, 71, 1253.
doi: 10.1103/RevModPhys.71.1253 |
|
| [22] |
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, Jr., J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, N. J.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, Ö.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J.Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford, CT, 2013.
|
| [23] |
(a) Becke, A. D. J. Chem. Phys. 1993, 98, 5648.
doi: 10.1063/1.464913 pmid: 9944570 |
|
(b) Stephens, P. J.; Devlin, F. J.; Chabalowski, C. F.; Frisch, M. J. J. Phys. Chem. 1994, 98, 11623.
doi: 10.1021/j100096a001 pmid: 9944570 |
|
|
(c) Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B: Condens. Matter Mater. Phys. 1988, 37, 785.
pmid: 9944570 |
|
| [24] |
(a) Dolg, M.; Stoll, H.; Preuss, H. J. Chem. Phys. 1989, 90, 1730.
doi: 10.1063/1.456066 |
|
(b) Dolg, M.; Wedig, U.; Stoll, H.; Preuss, H. J. Chem. Phys. 1987, 86, 866.
doi: 10.1063/1.452288 |
|
| [25] |
(a) Francl, M. M. J. Chem. Phys. 1982, 77, 3654.
doi: 10.1063/1.444267 |
|
(b) Hehre, W. J. J. Chem. Phys. 1972, 56, 2257.
doi: 10.1063/1.1677527 |
|
|
(c) Hariharan, P. C.; Pople, J. A. Theor. Chim. Acta 1973, 28, 213.
doi: 10.1007/BF00533485 |
|
| [26] |
(a) Sang-Aroon, W.; Ruangpornvisuti, V. Int. J. Quantum Chem. 2008, 108, 1181.
doi: 10.1002/qua.21569 |
|
(b) Tomasi, J.; Mennucci, B.; Cancès, E. J. Mol. Struct. 1999, 464, 211.
doi: 10.1016/S0166-1280(98)00553-3 |
|
| [27] |
(a) Gonzalez, C.; Schlegel, H. B. J. Chem. Phys. 1989, 90, 2154.
doi: 10.1063/1.456010 |
|
(b) Gonzalez, C.; Schlegel, H. B. J. Phys. Chem. 1990, 94, 5523.
doi: 10.1021/j100377a021 |
|
| [28] |
Zhao, Y.; Truhlar, D. Theor. Chem. Acc. 2008, 120, 215.
doi: 10.1007/s00214-007-0310-x |
| [29] |
(a) Weigend, F.; Ahlrichs, R. Phys. Chem. Chem. Phys. 2005, 7, 3297.
doi: 10.1039/b508541a |
|
(b) Cancès, E.; Mennucci, B.; Tomasi, J. J. Chem. Phys. 1997, 107, 3032.
doi: 10.1063/1.474659 |
|
| [30] |
(a) Liu, C.; Li, S.-J.; Han, P.; Qu, L.-B.; Lan, Y. Mol. Catal. 2021, 499, 111318.
pmid: 24787037 |
|
(b) Shen, B.; Liu, S.; Zhu, L.; Zhong, K.; Liu, F.; Chen, H.; Bai, R.; Lan, Y. Organometallics, 2020, 39, 2813.
doi: 10.1021/acs.organomet.0c00248 pmid: 24787037 |
|
|
(c) Wang, Y.; Liao, W.; Huang, G.; Xia, Y.; Yu, Z.-X. J. Org. Chem. 2014, 79, 5684.
doi: 10.1021/jo500839c pmid: 24787037 |
| [1] | 熊威, 石斌, 姜烜, 陆良秋, 肖文精. 配体调控钯催化乙烯基环状碳酰胺和异氰酸酯的差异性转化[J]. 有机化学, 2023, 43(1): 265-273. |
| [2] | 高秋珊, 李蒙, 伍婉卿. 过渡金属催化的异腈插入反应研究进展[J]. 有机化学, 2022, 42(9): 2659-2681. |
| [3] | 崔银, 张国富, 丁成荣. 洛森重排反应研究进展[J]. 有机化学, 2022, 42(7): 2015-2027. |
| [4] | 马丽文, 魏晓叶, 赵紫琳, 赵昂, 邓祥文, 霍丙南, 马刚, 张春芳. 端炔偶联反应中铜变价催化机制的理论研究[J]. 有机化学, 2022, 42(6): 1811-1819. |
| [5] | 徐曼, 夏远志. 铑(III)催化N-苯氧基乙酰胺与亚甲基氧杂环丁酮氧化还原中性的碳氢活化/环化反应的机理研究[J]. 有机化学, 2021, 41(8): 3272-3278. |
| [6] | 马蔚青, 韩莹, 孙晶, 颜朝国. 三组分反应高效合成螺[环戊烷-1,3'-吲哚啉]衍生物[J]. 有机化学, 2021, 41(8): 3180-3191. |
| [7] | 张明亮, 赵聘, 袁浩, 张安安, 张文宇, 郑琳琳, 吴冬青, 刘澜涛. 银催化的3-三氟乙酰基吲哚与异氰酸酯反应: (Z)-β-三氟甲基取代的脱氢色氨酸衍生物的高效合成[J]. 有机化学, 2021, 41(2): 669-676. |
| [8] | 邬林洋, 钟大猷, 刘文博. 无配体参与的铁催化分子内C(sp3)—H键胺化合成咪唑啉酮[J]. 有机化学, 2021, 41(10): 4083-4087. |
| [9] | 仝明慧, 张欣宇, 王也铭, 王自坤. 碘叶立德的化学反应研究进展[J]. 有机化学, 2021, 41(1): 126-143. |
| [10] | 王飞雨, 张志朋, 黄菲. 基于重氮酯的O—H插入反应研究进展[J]. 有机化学, 2021, 41(1): 144-157. |
| [11] | 吴燕, 王珊, 张海玲, 陈睿, 何树华. 铯催化吲哚衍生物N-酰胺化反应制备吲哚-1-芳(烷)基甲酰胺化合物[J]. 有机化学, 2019, 39(12): 3567-3573. |
| [12] | 吴锦雯, 朱佳雯, 李慧, 吴春雷, 沈润溥, 余乐茂. 过渡金属催化氧气氧插入反应研究进展[J]. 有机化学, 2019, 39(12): 3328-3337. |
| [13] | 封佳俊, 易享炎, 傅耀锋, 于杨, 黄菲. 基于α-羰基重氮化合物参与的N-H插入反应的研究进展[J]. 有机化学, 2019, 39(11): 3013-3025. |
| [14] | 李志娟, 翦辉, 王伟华, 王强, 何林. 苯炔与稳定硫叶立德插入反应[J]. 有机化学, 2018, 38(8): 2045-2053. |
| [15] | 罗大云, 崔时胜, 胡兴梅, 林军, 严胜骄. 酰胺类杂环烯酮缩胺的合成[J]. 有机化学, 2017, 37(1): 166-175. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||