有机化学 ›› 2022, Vol. 42 ›› Issue (6): 1722-1734.DOI: 10.6023/cjoc202112023 上一篇 下一篇
研究论文
王兴a, 宋倩倩a, 陈续玲b, 李鹏飞b,*( ), 齐昀坤a,*(
), 齐昀坤a,*( ), 李文军a,*(
), 李文军a,*( )
)
收稿日期:2021-12-18
修回日期:2022-02-28
发布日期:2022-03-08
通讯作者:
李鹏飞, 齐昀坤, 李文军
作者简介:基金资助:
Xing Wanga, Qianqian Songa, Xuling Chenb, Pengfei Lib( ), Yunkun Qia(
), Yunkun Qia( ), Wenjun Lia(
), Wenjun Lia( )
)
Received:2021-12-18
Revised:2022-02-28
Published:2022-03-08
Contact:
Pengfei Li, Yunkun Qi, Wenjun Li
About author:Supported by:文章分享

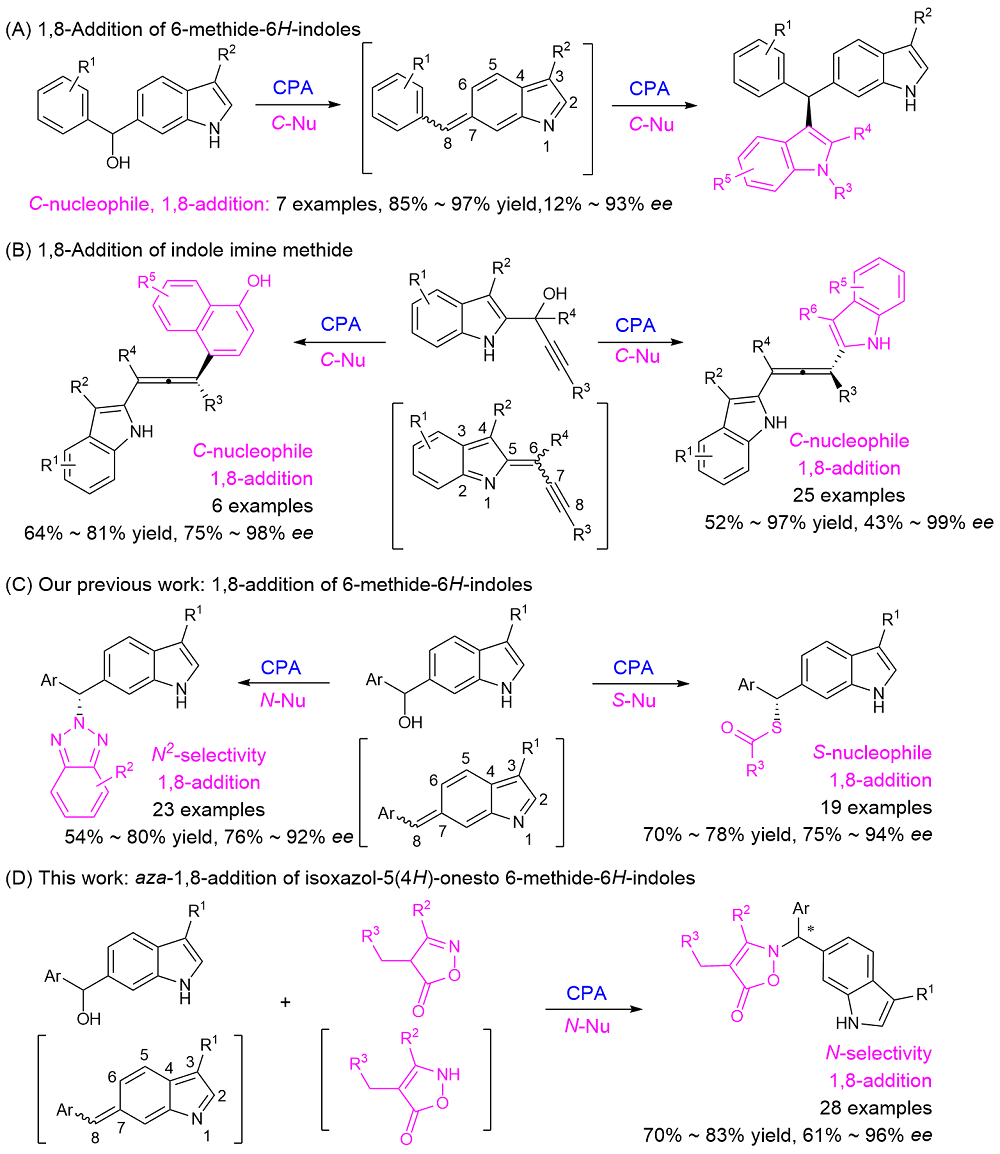
报道了在手性磷酸作用下6-吲哚甲醇原位生成6-亚甲基-6H-吲哚, 继而与异噁唑-5(4H)-酮发生不对称1,8-共轭加成反应. 该反应实现了异噁唑-5(4H)-酮的对映选择性N-烷基化, 以70%~83%的产率和61%~96%的对映选择性得到共轭加成产物.
王兴, 宋倩倩, 陈续玲, 李鹏飞, 齐昀坤, 李文军. 有机催化远程立体控制6-亚甲基-6H-吲哚与异噁唑-5(4H)-酮的氮杂1,8-共轭加成反应[J]. 有机化学, 2022, 42(6): 1722-1734.
Xing Wang, Qianqian Song, Xuling Chen, Pengfei Li, Yunkun Qi, Wenjun Li. Organocatalytic Regio- and Enantioselective aza-1,8-Conjugate Additions of Isoxazol-5(4H)-ones to 6-Methide-6H-indoles[J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1722-1734.
| Entry | Catalyst | Solvent | Yieldb/% | eec/% |
|---|---|---|---|---|
| 1 | CPA-1 | CH2Cl2 | 3aa, 75 | ≈28 |
| 2 | CPA-2 | CH2Cl2 | 3aa, 74 | ≈53 |
| 3 | CPA-3 | CH2Cl2 | 3aa, 62 | ≈66 |
| 4 | CPA-4 | CH2Cl2 | 3aa, 57 | ≈68 |
| 5 | CPA-5 | CH2Cl2 | 3aa, 77 | 88 |
| 6 | CPA-6 | CH2Cl2 | 3aa, 49 | 48 |
| 7 | CPA-5 | CHCl3 | 3aa, 80 | 68 |
| 8 | CPA-5 | THF | 3aa, 68 | 43 |
| 9 | CPA-5 | Et2O | 3aa, 65 | 49 |
| 10 | CPA-5 | Toluene | 3aa, 77 | 76 |
| 11 | CPA-5 | PhCF3 | 3aa, 69 | 90 |
| 12 | CPA-5 | Xylenes | 3aa, 70 | 96 |
| 13d | CPA-5 | Xylenes | 3aa, 81 | 96 |
| 14e | CPA-5 | Xylenes | 3aa, 74 | 92 |
| 15f | CPA-5 | Xylenes | 3aa, 83 | 94 |
| Entry | Catalyst | Solvent | Yieldb/% | eec/% |
|---|---|---|---|---|
| 1 | CPA-1 | CH2Cl2 | 3aa, 75 | ≈28 |
| 2 | CPA-2 | CH2Cl2 | 3aa, 74 | ≈53 |
| 3 | CPA-3 | CH2Cl2 | 3aa, 62 | ≈66 |
| 4 | CPA-4 | CH2Cl2 | 3aa, 57 | ≈68 |
| 5 | CPA-5 | CH2Cl2 | 3aa, 77 | 88 |
| 6 | CPA-6 | CH2Cl2 | 3aa, 49 | 48 |
| 7 | CPA-5 | CHCl3 | 3aa, 80 | 68 |
| 8 | CPA-5 | THF | 3aa, 68 | 43 |
| 9 | CPA-5 | Et2O | 3aa, 65 | 49 |
| 10 | CPA-5 | Toluene | 3aa, 77 | 76 |
| 11 | CPA-5 | PhCF3 | 3aa, 69 | 90 |
| 12 | CPA-5 | Xylenes | 3aa, 70 | 96 |
| 13d | CPA-5 | Xylenes | 3aa, 81 | 96 |
| 14e | CPA-5 | Xylenes | 3aa, 74 | 92 |
| 15f | CPA-5 | Xylenes | 3aa, 83 | 94 |
| Entrya | Ar | R1 | Yieldb/% | eec/% |
|---|---|---|---|---|
| 1 | 4-MeOC4H6 | Ph | 3aa, 81 | 96 |
| 2d | 4-MeC4H6 | Ph | 3ba, 76 | 96 |
| 3 | 4-FC4H6 | Ph | 3ca, 77 | 96 |
| 4 | 3-MeOC4H6 | Ph | 3da, 70 | 80 |
| 5 | 3-ClC4H6 | Ph | 3ea, 70 | 84 |
| 6 | 2-MeC4H6 | Ph | 3fa, 79 | 67 |
| 7d | Ph | Ph | 3ga, 75 | 77 |
| 8 | 4-MeOC4H6 | 4-MeOC4H6 | 3ha, 81 | 94 |
| 9 | 4-MeOC4H6 | 2-MeOC4H6 | 3ia, 81 | 92 |
| 10 | 4-MeOC4H6 | 2-Naphthyl | 3ja, 74 | 95 |
| 11 | 4-MeOC4H6 | H | 3ka, 70 | 75 |
| 12 | 4-MeOC4H6 | Me | 3la, 79 | 65 |
| Entrya | Ar | R1 | Yieldb/% | eec/% |
|---|---|---|---|---|
| 1 | 4-MeOC4H6 | Ph | 3aa, 81 | 96 |
| 2d | 4-MeC4H6 | Ph | 3ba, 76 | 96 |
| 3 | 4-FC4H6 | Ph | 3ca, 77 | 96 |
| 4 | 3-MeOC4H6 | Ph | 3da, 70 | 80 |
| 5 | 3-ClC4H6 | Ph | 3ea, 70 | 84 |
| 6 | 2-MeC4H6 | Ph | 3fa, 79 | 67 |
| 7d | Ph | Ph | 3ga, 75 | 77 |
| 8 | 4-MeOC4H6 | 4-MeOC4H6 | 3ha, 81 | 94 |
| 9 | 4-MeOC4H6 | 2-MeOC4H6 | 3ia, 81 | 92 |
| 10 | 4-MeOC4H6 | 2-Naphthyl | 3ja, 74 | 95 |
| 11 | 4-MeOC4H6 | H | 3ka, 70 | 75 |
| 12 | 4-MeOC4H6 | Me | 3la, 79 | 65 |
| Entrya | R2 | R3 | 3, Yieldb/% | eec/% |
|---|---|---|---|---|
| 1 | Ph | 4-MeOC4H6 | 3ab, 82 | 92 |
| 2 | Ph | 4-ClC4H6 | 3ac, 70 | 89 |
| 3 | Ph | 4-BrC4H6 | 3ad, 79 | 94 |
| 4 | Ph | 4-NO2C4H6 | 3ae, 74 | 90 |
| 5 | Ph | 3-MeC4H6 | 3af, 79 | 96 |
| 6 | Ph | 3-ClC4H6 | 3ag, 79 | 92 |
| 7 | Ph | 3-BrC4H6 | 3ah, 79 | 96 |
| 8 | Ph | 2-MeC4H6 | 3ai, 77 | 94 |
| 9 | Ph | 2-BrC4H6 | 3aj, 77 | 86 |
| 10 | Ph | 2-Naphthyl | 3ak, 83 | 90 |
| 11 | Ph | 1-Naphthyl | 3al, 79 | 96 |
| 12 | Ph | 2-Furyl | 3am, 80 | 66 |
| 13d | Ph | H | 3an, 75 | 63 |
| 14 | Me | Ph | 3ao, 81 | 61 |
| 15e | Et | Ph | 3ap, 72 | 70 |
| 16 | Ph | Ph | 3aq, 82 | 84 |
| Entrya | R2 | R3 | 3, Yieldb/% | eec/% |
|---|---|---|---|---|
| 1 | Ph | 4-MeOC4H6 | 3ab, 82 | 92 |
| 2 | Ph | 4-ClC4H6 | 3ac, 70 | 89 |
| 3 | Ph | 4-BrC4H6 | 3ad, 79 | 94 |
| 4 | Ph | 4-NO2C4H6 | 3ae, 74 | 90 |
| 5 | Ph | 3-MeC4H6 | 3af, 79 | 96 |
| 6 | Ph | 3-ClC4H6 | 3ag, 79 | 92 |
| 7 | Ph | 3-BrC4H6 | 3ah, 79 | 96 |
| 8 | Ph | 2-MeC4H6 | 3ai, 77 | 94 |
| 9 | Ph | 2-BrC4H6 | 3aj, 77 | 86 |
| 10 | Ph | 2-Naphthyl | 3ak, 83 | 90 |
| 11 | Ph | 1-Naphthyl | 3al, 79 | 96 |
| 12 | Ph | 2-Furyl | 3am, 80 | 66 |
| 13d | Ph | H | 3an, 75 | 63 |
| 14 | Me | Ph | 3ao, 81 | 61 |
| 15e | Et | Ph | 3ap, 72 | 70 |
| 16 | Ph | Ph | 3aq, 82 | 84 |
| [5] |
Beccalli, E. M.; Marchesini, A. Synthesis 1991, 861.
|
| [6] |
(a) Okamoto, K.; Oda, T.; Kohigashi, S.; Ohe, K. Angew. Chem., Int. Ed. 2011, 50, 11470.
doi: 10.1002/anie.201105153 pmid: 28832162 |
|
(b) Okamoto, K.; Shimbayashi, T.; Yoshida, M.; Nanya, A.; Ohe, K. Angew. Chem., Int. Ed. 2016, 55, 7199.
doi: 10.1002/anie.201602241 pmid: 28832162 |
|
|
(c) Rieckhoff, S.; Titze, M.; Frey, W.; Peters, R. Org. Lett. 2017, 19, 4436.
doi: 10.1021/acs.orglett.7b01895 pmid: 28832162 |
|
| [7] |
Okamoto, K.; Shimbayashi, T.; Tamura, E.; Ohe, K. Chem. Eur. J. 2014, 20, 1490.
doi: 10.1002/chem.201304211 |
| [8] |
(a) Rieckhoff, S.; Hellmuth, T.; Peters, R. J. Org. Chem. 2015, 80, 6822.
doi: 10.1021/acs.joc.5b01065 pmid: 26101943 |
|
(b) Fernandes, A. A. G.; Stivanin, M. L.; Jurberg, I. D. ChemistrySelect 2019, 4, 3360.
doi: 10.1002/slct.201900761 pmid: 26101943 |
|
| [9] |
Rieckhoff, S.; Frey, W.; Peters, R. Eur. J. Org. Chem. 2018, 1797.
|
| [10] |
(a) Liang, H.-W.; Yang, Z.; Jiang, K.; Ye, J.; Wei, Y. Angew. Chem., Int. Ed. 2018, 57, 5720.
doi: 10.1002/anie.201801363 |
|
(b) Lang, J.; Wei, Y. Synlett 2019, 30, 252.
doi: 10.1055/s-0037-1610348 |
|
| [11] |
(a) Zhao, T.; Zhang, H.; Cui, L.; Wang, C.; Qu, J.; Wang, B. ChemistrySelect 2016, 1, 3713.
doi: 10.1002/slct.201600823 pmid: 32182085 |
|
(b) Shimbayashi, T.; Matsushita, G.; Nanya, A.; Eguchi, A.; Okamoto, K.; Ohe, K. ACS Catal. 2018, 8, 7773.
doi: 10.1021/acscatal.8b01646 pmid: 32182085 |
|
|
(c) Christodoulou, M. S.; Giofrè, S.; Beccalli, E. M.; Foschi, F.; Broggini, G. Org. Lett. 2020, 22, 2735.
doi: 10.1021/acs.orglett.0c00709 pmid: 32182085 |
|
|
(d) Wang, T.; Pi, C.; Wu, Y.; Cui, X. Org. Lett. 2020, 22, 6484.
doi: 10.1021/acs.orglett.0c02283 pmid: 32182085 |
|
| [12] |
(a) Meng, W.-T.; Zheng, Y.; Nie, J.; Xiong, H.-Y.; Ma, J.-A. J. Org. Chem. 2013, 78, 559.
doi: 10.1021/jo302419e pmid: 29240444 |
|
(b) Hellmuth, T.; Frey, W.; Peters, R. Angew. Chem., Int. Ed. 2015, 54, 2788.
doi: 10.1002/anie.201410933 pmid: 29240444 |
|
|
(c) Zhang, H.; Wang, B.; Cui, L.; Bao, X.; Qu, J.; Song, Y. Eur. J. Org. Chem. 2015, 2143.
pmid: 29240444 |
|
|
(d) Rieckhoff, S.; Meisner, J.; Kästner, J.; Frey, W.; Peters, R. Angew. Chem., Int. Ed. 2018, 57, 1404.
doi: 10.1002/anie.201710940 pmid: 29240444 |
|
|
(e) Xiao, W.; Zhou, Z.; Yang, Q.-Q.; Du, W.; Chen, Y.-C. Adv. Synth. Catal. 2018, 360, 3526.
doi: 10.1002/adsc.201800636 pmid: 29240444 |
|
|
(f) Xiao, W.; Yang, Q.-Q.; Chen, Z.; Ouyang, Q.; Du, W.; Chen, Y.-C. Org. Lett. 2018, 20, 236.
doi: 10.1021/acs.orglett.7b03598 pmid: 29240444 |
|
| [13] |
Torán, R.; Vila, C.; Sanz-Marco, A.; Muñoz, M. C.; Pedro, J. R.; Blay, G. Eur. J. Org. Chem. 2020, 627.
|
| [14] |
Qi, S.-S.; Jiang, Z.-H.; Chu, M.-M.; Wang, Y.-F.; Chen, X.-Y.; Ju, W.-Z.; Xu, D.-Q. Org. Biomol. Chem. 2020, 18, 2398.
doi: 10.1039/D0OB00393J |
| [15] |
For selected reviews, see: (b) Fuson, R. C. Chem. Rev. 1935, 16, 1.
doi: 10.1021/cr60053a001 |
|
(b) Jiang, H.; Albrecht, Ł.; Jørgenson, K. A. Chem. Sci. 2013, 4, 2287.
doi: 10.1039/c3sc50405k |
|
|
(c) Curti, C.; Battistini, L.; Sartori, A.; Zanardi, F. Chem. Rev. 2020, 120, 2448.
doi: 10.1021/acs.chemrev.9b00481 |
|
| [16] |
Uraguchi, D.; Yoshioka, K.; Ueki, Y.; Ooi, T. J. Am. Chem. Soc. 2012, 134, 19370.
doi: 10.1021/ja310209g pmid: 23145913 |
| [17] |
(a) Qian, D.; Wu, L.; Lin, Z.; Sun, J. Nat. Commun. 2017, 8, 567.
doi: 10.1038/s41467-017-00251-x pmid: 31544464 |
|
(b) Chen, M.; Qian, D.; Sun, J. Org. Lett. 2019, 21, 8127.
doi: 10.1021/acs.orglett.9b03224 pmid: 31544464 |
|
| [18] |
Liu, X.; Zhang, J.; Bai, L.; Wang, L.; Yang, D.; Wang, R. Chem. Sci. 2020, 11, 671.
doi: 10.1039/C9SC05320D |
| [19] |
(a) Zhang, P.; Huang, Q.; Cheng, Y.; Li, R.; Li, P.; Li, W. Org. Lett. 2019, 21, 503.
doi: 10.1021/acs.orglett.8b03801 pmid: 31486650 |
|
(b) Zhang, L.; Han, Y.; Huang, A.; Zhang, P.; Li, P.; Li, W. Org. Lett. 2019, 21, 7415.
doi: 10.1021/acs.orglett.9b02726 pmid: 31486650 |
|
| [20] |
Yue, C.; Na, F.; Fang, X.; Cao, Y.; Antilla, J. C. Angew. Chem., Int. Ed. 2018, 57, 11004.
doi: 10.1002/anie.201804330 |
| [21] |
Li, X.; Sun, J. Angew. Chem., Int. Ed. 2020, 59, 17049.
doi: 10.1002/anie.202006137 |
| [22] |
(a) Li, W.; Xu, X.; Liu, Y.; Gao, H.; Cheng, Y.; Li, P. Org. Lett. 2018, 20, 1142.
doi: 10.1021/acs.orglett.8b00072 |
| [1] |
da Silva, A. F.; Fernandes, A. A. G.; Thurow, S.; Stivanin, M. L.; Jurberg, I. D. Synthesis 2018, 50, 2473.
doi: 10.1055/s-0036-1589534 |
| [2] |
(a) Beccalli, E. M.; Rosa, C. L.; Marchesini, A. J. Org. Chem. 1984, 49, 4287.
doi: 10.1021/jo00196a034 pmid: 28892631 |
|
(b) Jurberg, I. D.; Davies, H. M. L. Org. Lett. 2017, 19, 5158.
doi: 10.1021/acs.orglett.7b02436 pmid: 28892631 |
|
|
(c) Zhu, Y.-M.; Zhang, W.; Li, H.; Xu, X.-P.; Ji, S.-J. Adv. Synth. Catal. 2021, 363, 808.
doi: 10.1002/adsc.202001200 pmid: 28892631 |
|
| [3] |
(a) Boivin, J.; Elkaim, L.; Ferro, P. G.; Zard, S. Z. Tetrahedron Lett. 1991, 32, 5321.
doi: 10.1016/S0040-4039(00)92375-X |
|
(b) Boivin, J.; Huppé, S.; Zard, S. Z. Tetrahedron Lett. 1996, 37, 8735.
doi: 10.1016/S0040-4039(96)02015-1 |
|
|
(c) Boutillier, P.; Zard, S. Z. Chem. Commun. 2001, 1304.
|
|
| [4] |
Beccalli, E. M.; Marchesini, A.; Pilati, T. Synthesis 1991, 127.
|
| [22] |
(b) Li, W.; Yuan, H.; Liu, Z.; Zhang, Z.; Cheng, Y.; Li, P. Adv. Synth. Catal. 2018, 360, 2460.
doi: 10.1002/adsc.201800337 |
|
(c) Li, F.; Chen, X.; Liang, S.; Shi, Z.; Li, P.; Li, W. Org. Chem. Front. 2020, 7, 3446.
doi: 10.1039/D0QO00888E |
|
|
(d) Song, Q.; Zhang, P.; Liang, S.; Chen, X.; Li, P.; Li, W. Org. Lett. 2020, 22, 7859.
doi: 10.1021/acs.orglett.0c02769 |
|
| [23] |
(a) Li, P.; Fang, F.; Chen, J.; Wang, J. Tetrahedron: Asymmetry 2014, 25, 98.
pmid: 31486458 |
|
(b) Fang, F.; Hua, G.; Shi, F.; Li, P. Org. Biomol. Chem. 2015, 13, 4395.
doi: 10.1039/c5ob00175g pmid: 31486458 |
|
|
(c) Huang, Q.; Zhang, L.; Cheng, Y.; Li, P.; Li, W. Adv. Synth. Catal. 2018, 360, 3266.
doi: 10.1002/adsc.201800642 pmid: 31486458 |
|
|
(d) Huang, Q.; Cheng, Y.; Yuan, H.; Chang, X.; Li, P.; Li, W. Org. Chem. Front. 2018, 5, 3226.
doi: 10.1039/C8QO00814K pmid: 31486458 |
|
|
(e) Qian, C.; Zhang, P.; Li, W.; Li, P. Asian J. Org. Chem. 2019, 8, 242.
pmid: 31486458 |
|
|
(f) Zhang, C.; Shang, X.; Cheng, Y.; Li, F.; Zhao, H.; Li, P.; Li, W. Org. Biomol. Chem. 2019, 17, 8374.
doi: 10.1039/c9ob01870k pmid: 31486458 |
|
|
(g) Li, F.; Yang, Z.; Yang, Y.; Huang, Q.; Chen, X.; Li, P.; Dong, M.; Li, W. Adv. Synth. Catal. 2021, 363, 2557.
doi: 10.1002/adsc.202100039 pmid: 31486458 |
|
| [24] |
For selected recent reviews, see: (a) Zhang Y.-C.; Jiang, F.; Shi, F. Acc. Chem. Res. 2020, 53, 425.
doi: 10.1021/acs.accounts.9b00549 pmid: 32720968 |
|
(b) Zeng, L.; Lin, Y.; Cui, S. Chem. Asian J. 2020, 15, 973.
doi: 10.1002/asia.201901806 pmid: 32720968 |
|
|
(c) Pradhan, S.; De, P. B.; Shah, T. A.; Punniyamurthy, T. Chem. Asian J. 2020, 15, 4184.
doi: 10.1002/asia.202001159 pmid: 32720968 |
|
|
(d) Li, T.-Z.; Liu, S.-J.; Tan, W.; Shi, F. Chem. Eur. J. 2020, 26, 15779.
doi: 10.1002/chem.202001397 pmid: 32720968 |
|
|
(e) Cerveri, A.; Bandini, M. Chin. J. Chem. 2020, 38, 287.
doi: 10.1002/cjoc.201900446 pmid: 32720968 |
|
|
(f) Alonso, J. M.; Muñoz, M. P. Eur. J. Org. Chem. 2020, 7197.
pmid: 32720968 |
|
|
(g) Milcendeau, P.; Sabat, N.; Ferry, A.; Guinchard, X. Org. Biomol. Chem. 2020, 18, 6006.
doi: 10.1039/d0ob01245a pmid: 32720968 |
|
|
(h) Bonandi, E.; Perdicchia, D.; Colombo, E.; Foschi, F.; Marzullo, P.; Passarella, D. Org. Biomol. Chem. 2020, 18, 6211.
doi: 10.1039/d0ob01094d pmid: 32720968 |
|
|
(i) Sheng, F.-T.; Wang, J.-Y.; Tan, W.; Zhang, Y.-C.; Shi, F. Org. Chem. Front. 2020, 7, 3967.
doi: 10.1039/D0QO01124J pmid: 32720968 |
|
|
(j) Deka, B.; Deb, M. L.; Baruah, P. K. Topics Curr. Chem. 2020, 378, 22.
doi: 10.1007/s41061-020-0287-7 pmid: 32720968 |
|
|
(k) Neto, J.; Zeni, G. Org. Chem. Front. 2020, 7, 155.
doi: 10.1039/C9QO01315F pmid: 32720968 |
|
|
(l) Tu, M.; Chen, K.; Wu, P.; Zhang, Y.; Liu, X.; Shi, F. Org. Chem. Front. 2021, 8, 2643.
doi: 10.1039/D0QO01643H pmid: 32720968 |
|
| [25] |
For selected reviews on CPAs, see: (a) Akiyama, T. Chem. Rev. 2007, 107, 5744.
doi: 10.1021/cr068374j pmid: 25203602 |
|
(b) Terada, M. Synthesis 2010, 12, 1929.
pmid: 25203602 |
|
|
(c) Yu, J.; Shi, F.; Gong, L.-Z. Acc. Chem. Res. 2011, 44, 1156.
doi: 10.1021/ar2000343 pmid: 25203602 |
|
|
(d) Parmar, D.; Sugiono, E.; Raja, S.; Rueping, M. Chem. Rev. 2014, 114, 9047.
doi: 10.1021/cr5001496 pmid: 25203602 |
|
|
(e) Wu, H.; He, Y.-P.; Shi, F. Synthesis 2015, 47, 1990.
doi: 10.1055/s-0034-1378837 pmid: 25203602 |
|
|
(f) Merad, J.; Lalli, C.; Bernadat, G.; Maury, J.; Masson, G. Chem. Eur. J. 2018, 24, 3925.
pmid: 25203602 |
| [1] | 陈文龙, 李慧敏, 杨鹏飞, 郑东程, 杨高升. 2-芳甲酰基甲亚基丙二酸酯与Corey叶立德的反应[J]. 有机化学, 2023, 43(4): 1472-1482. |
| [2] | 王东琳, 阚玲珑, 马玉道, 刘磊. 叔丁醇钠催化的δ-腈基-δ-芳基-双取代的对亚甲基苯醌和二芳基氧磷的磷氢化反应研究[J]. 有机化学, 2021, 41(8): 3192-3203. |
| [3] | 李华, 庞靖祥, 刘华铮, 赵长印, 李松, 王恒山, 刘希功. Sc(OTf)3催化的δ-三氟甲基-δ-芳基-双取代对亚甲基苯醌和硫醇的反应: 高效合成二芳基甲烷硫醚化合物[J]. 有机化学, 2021, 41(8): 3134-3143. |
| [4] | 张同飞, 陈逸波, 高振博. 三氟甲磺酸铜(II)催化的硫醇对烯酮的共轭加成反应[J]. 有机化学, 2021, 41(6): 2424-2434. |
| [5] | 余述燕, 高丽宏, 兰宏兵, 钱恒玉, 尹志刚, 商永嘉. 橙酮衍生氮杂二烯的化学反应进展[J]. 有机化学, 2020, 40(9): 2714-2724. |
| [6] | 王琳, 王楠, 齐越, 孙书涛, 刘希功, 李伟, 刘磊. 基于δ-腈基取代对亚甲基苯醌1,6-氮杂共轭加成的大位阻α-氰胺合成研究[J]. 有机化学, 2020, 40(11): 3934-3943. |
| [7] | 林晔, 杜大明. 方酰胺催化的不对称串联反应合成螺环氧吲哚衍生物研究进展[J]. 有机化学, 2020, 40(10): 3214-3236. |
| [8] | 刘腾, 刘建军, 贺池先, 成飞翔. 多共轭硝基二烯炔/硝基烯炔的合成以及应用研究进展[J]. 有机化学, 2017, 37(10): 2609-2618. |
| [9] | 陈学伟, 辛玉娟, 刘青, 兰支利. 含辅助基的双功能(硫)脲催化剂在不对称有机催化中的应用[J]. 有机化学, 2016, 36(2): 306-314. |
| [10] | 邢爱萍, 田密, 王来来. C3对称的新型单齿亚磷酸酯配体在不对称氢甲酰化和1,4-共轭加成中的应用研究[J]. 有机化学, 2016, 36(12): 2912-2919. |
| [11] | 唐凤翔, 叶久勇. 铜催化格氏试剂不对称共轭加成研究进展[J]. 有机化学, 2015, 35(7): 1414-1427. |
| [12] | 林敬, 程宇, 康泰然, 何龙, 刘全忠. 不饱和酮亚胺叶立德的分子内共轭加成[J]. 有机化学, 2014, 34(4): 735-740. |
| [13] | 倪承燕, 李文科, 何龙, 刘全忠, 康泰然. 二乙基锌对原位产生吲哚亚胺的对映选择性加成反应[J]. 有机化学, 2012, 32(12): 2322-2327. |
| [14] | 徐涛, 贡卫涛, 叶俊伟, 宁桂玲, 林源. 2,4,6-三苯基吡喃盐与三烷基(芳基)膦的新型共轭加成反应[J]. 有机化学, 2011, 31(10): 1707-1709. |
| [15] | 于艳飞 姜岚 李争宁 单凤军. 铜-手性氮杂环卡宾化合物催化的不对称1,4-共轭加成反应[J]. 有机化学, 2011, 31(04): 443-452. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||