有机化学 ›› 2021, Vol. 41 ›› Issue (8): 3134-3143.DOI: 10.6023/cjoc202103042 上一篇 下一篇
研究论文
李华a, 庞靖祥c, 刘华铮b, 赵长印b, 李松b, 王恒山a,*( ), 刘希功b,*(
), 刘希功b,*( )
)
收稿日期:2021-03-23
修回日期:2021-04-19
发布日期:2021-05-25
通讯作者:
王恒山, 刘希功
作者简介:基金资助:
Hua Lia, Jingxiang Pangc, Huazheng Liub, Changyin Zhaob, Song Lib, Hengshan Wanga( ), Xigong Liub(
), Xigong Liub( )
)
Received:2021-03-23
Revised:2021-04-19
Published:2021-05-25
Contact:
Hengshan Wang, Xigong Liu
About author:Supported by:文章分享
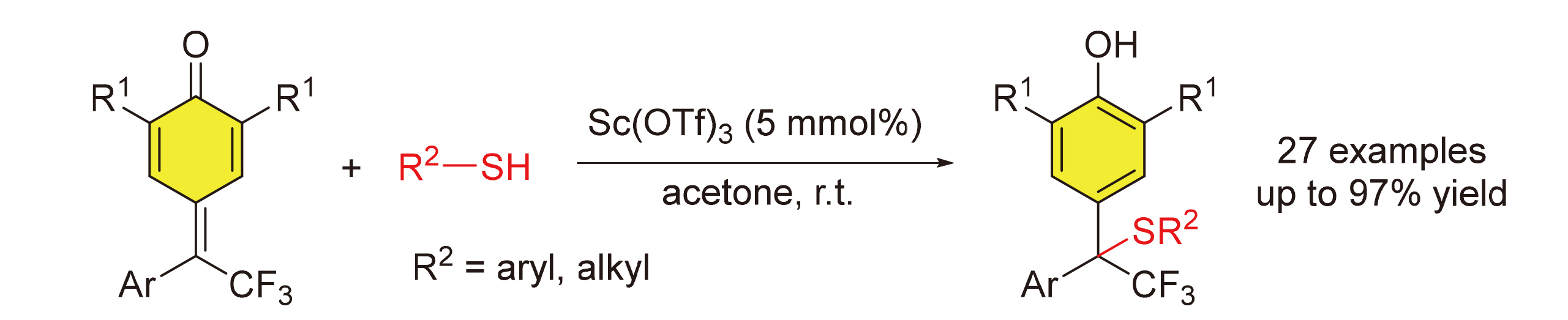
报道了一种δ-三氟甲基-δ-芳基-二取代的对亚甲基苯醌和硫醇参与的1,6-共轭加成反应. 在5 mol%三氟甲基磺酸钪催化的条件下, 得到了结构多样化的含有三氟甲基四级碳中心的二芳基甲烷硫醚. 反应具有良好的官能团兼容性和底物范围. 此外, 烷基硫醇和苄硫醇也适用于该反应. 鉴于二芳基甲烷硫醚骨架以及三氟甲基在生物活性分子中的重要性, 发展的含有三氟甲基取代四级碳中心的二芳基甲烷硫醚的高效合成方法将为生物活性分子的发现提供一条简洁、高效的策略.
李华, 庞靖祥, 刘华铮, 赵长印, 李松, 王恒山, 刘希功. Sc(OTf)3催化的δ-三氟甲基-δ-芳基-双取代对亚甲基苯醌和硫醇的反应: 高效合成二芳基甲烷硫醚化合物[J]. 有机化学, 2021, 41(8): 3134-3143.
Hua Li, Jingxiang Pang, Huazheng Liu, Changyin Zhao, Song Li, Hengshan Wang, Xigong Liu. Sc(OTf)3-Catalyzed 1,6-Conjugate Addition of Thiols to δ-CF3-δ-aryl-disubstituted para-Quinone Methides: Efficient Construction of Diarylmethane Thioethers[J]. Chinese Journal of Organic Chemistry, 2021, 41(8): 3134-3143.
| Entry | Catalyst | Solvent | Time/h | Yieldb |
|---|---|---|---|---|
| 1 | — | Acetone | 24 | <5 |
| 2 | PTSA | Acetone | 5 | 85 |
| 3 | TFA | Acetone | 24 | 69 |
| 4 | (PhO)2POOH | Acetone | 12 | 75 |
| 5 | Fe(OTf)2 | Acetone | 48 | 46 |
| 6 | Fe(OTf)3 | Acetone | 24 | 70 |
| 7 | Cu(OTf)2 | Acetone | 24 | 75 |
| 8 | Sc(OTf)3 | Acetone | 0.1 | 93 |
| 9 | Bi(OTf)3 | Acetone | 12 | 88 |
| 10 | AgOTf | Acetone | 12 | 50 |
| 11 | Sc(OTf)3 | CH2Cl2 | 0.3 | 91 |
| 12 | Sc(OTf)3 | CH2ClCH2Cl | 0.3 | 91 |
| 13 | Sc(OTf)3 | THF | 0.3 | 90 |
| 14 | Sc(OTf)3 | EtOAc | 05 | 87 |
| 15 | Sc(OTf)3 | Toluene | 0.3 | 91 |
| Entry | Catalyst | Solvent | Time/h | Yieldb |
|---|---|---|---|---|
| 1 | — | Acetone | 24 | <5 |
| 2 | PTSA | Acetone | 5 | 85 |
| 3 | TFA | Acetone | 24 | 69 |
| 4 | (PhO)2POOH | Acetone | 12 | 75 |
| 5 | Fe(OTf)2 | Acetone | 48 | 46 |
| 6 | Fe(OTf)3 | Acetone | 24 | 70 |
| 7 | Cu(OTf)2 | Acetone | 24 | 75 |
| 8 | Sc(OTf)3 | Acetone | 0.1 | 93 |
| 9 | Bi(OTf)3 | Acetone | 12 | 88 |
| 10 | AgOTf | Acetone | 12 | 50 |
| 11 | Sc(OTf)3 | CH2Cl2 | 0.3 | 91 |
| 12 | Sc(OTf)3 | CH2ClCH2Cl | 0.3 | 91 |
| 13 | Sc(OTf)3 | THF | 0.3 | 90 |
| 14 | Sc(OTf)3 | EtOAc | 05 | 87 |
| 15 | Sc(OTf)3 | Toluene | 0.3 | 91 |
| [1] |
(a) Kung, A. L.; Zabludoff, S. D.; France, D. S.; Freedman, S. J.; Tanner, E. A.; Vieira, A.; Cornell-Kennon, S.; Lee, J.; Wang, B.; Wang, J.; Memmert, K.; Naegeli, H.-U.; Petersen, F.; Eck, M. J.; Bair, K. W.; Wood, A. W.; Livingston, D. M. Cancer Cell 2004, 6, 33.
doi: 10.1016/j.ccr.2004.06.009 pmid: 11040275 |
|
(b) Yurek-George, A.; Habens, F.; Brimmell, M.; Packham, G.; Ganesan, A. J. Am. Chem. Soc. 2004, 126, 1030.
pmid: 11040275 |
|
|
(c) Jayakanthan, K.; Mohan, S.; Pinto, B. M. J. Am. Chem. Soc. 2009, 131, 5621.
doi: 10.1021/ja900867q pmid: 11040275 |
|
|
(d) Krutetskaya, Z. I.; Milenina, L. S.; Naumova, A. A.; Antonov, V. G.; Nozdrachev, A. D. Dokl. Biochem. Biophys. 2016, 469, 302.
doi: 10.1134/S1607672916040177 pmid: 11040275 |
|
|
(e) Muchmore, D. B. Oncologist 2000, 5, 388.
pmid: 11040275 |
|
| [2] |
(a) McCooey, S. H.; Connon, S. J. Angew. Chem. Int. Ed. 2005, 44, 6367.
doi: 10.1002/(ISSN)1521-3773 pmid: 21378935 |
|
(b) Okino, T.; Hoashi, Y.; Takemoto, Y. J. Am. Chem. Soc. 2003, 125, 12672.
doi: 10.1021/ja036972z pmid: 21378935 |
|
|
(c) Stang, E. M.; Christina White, M. Nat. Chem. 2009, 1, 547.
doi: 10.1038/nchem.351 pmid: 21378935 |
|
|
(d) Lai, H.; Huang, Z.; Wu, Q.; Qin, Y. J. Org. Chem. 2009, 74, 283.
doi: 10.1021/jo802036m pmid: 21378935 |
|
|
(e) Ji, Y.; Riera, A.; Verdaguer, X. Org. Lett. 2009, 11, 4346.
doi: 10.1021/ol901728r pmid: 21378935 |
|
|
(f) Fang, G. Y.; Wallner, O. A.; Di Blasio, N.; Ginesta, X. Harvey, J. N.; Aggarwal, V. K. J. Am. Chem. Soc. 2007, 129, 14632.
doi: 10.1021/ja074110i pmid: 21378935 |
|
|
(g) Unthank, M. G.; Tavassoli, B.; Aggarwal, V. K. Org. Lett. 2008, 10, 1501.
doi: 10.1021/ol800318h pmid: 21378935 |
|
| [3] |
(a) Devendar, P.; Yang, G.-F. Top. Curr. Chem. 2017, 375, 82.
|
|
(b) Dubbaka, S. R.; Vogel, P. Angew. Chem. Int. Ed. 2005, 44, 7674.
doi: 10.1002/(ISSN)1521-3773 |
|
|
(c) Cohen, A.; Crozet, M. D.; Rathelot, P.; Azas, N.; Vanelle, P. Molecules 2013, 18, 97.
doi: 10.3390/molecules18010097 |
|
| [4] |
(a) Kondo, T.; Mitsudo, T.-A. Chem. Rev. 2000, 100, 3205.
pmid: 11749318 |
|
(b) Lee, C.-F.; Liu, Y.-C.; Badsara, S. S. Chem.-Asian J. 2014, 9, 706.
doi: 10.1002/asia.v9.3 pmid: 11749318 |
|
|
(c) Saidhareddy, N. Wang, P.; Jiang, X. Nat. Prod. Rep. 2020, 37, 246.
doi: 10.1039/C8NP00093J pmid: 11749318 |
|
|
(d) Hosseinian, A.; Arshadi, S.; Sarhandi, S.; Monfared, A.; Vessally, E. J. Sulfur Chem. 2019, 40, 289.
doi: 10.1080/17415993.2019.1582654 pmid: 11749318 |
|
|
(e) Li, J.; Yang, S.; Wu, W. Jiang, H. Org. Chem. Front. 2020, 7, 1395.
doi: 10.1039/D0QO00377H pmid: 11749318 |
|
|
(f) Emmett, E. J.; Willis, M. C. Asian J. Org. Chem. 2015, 4, 602.
doi: 10.1002/ajoc.201500103 pmid: 11749318 |
|
| [5] |
(a) Kao, R. Y. T.; Jenkins, J. L.; Olson, K. A.; Key, M. E.; Fett, J. W.; Shapiro, R. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 10066.
doi: 10.1073/pnas.152342999 |
|
(b) Goyal, M.; Singh, P.; Alam, A.; Das, S. K.; Iqbal, M. S.; Dey, S.; Bindu, S.; Pal, C.; Das, S. K.; Panda, G.; Bandyopadhyay, U. Free Radical Biol. Med. 2012, 53, 129.
doi: 10.1016/j.freeradbiomed.2012.04.028 |
|
|
(d) Kumar, R. Drugs 2008, 68, 1803.
doi: 10.2165/00003495-200868130-00003 |
|
|
(e) Gouliaev, A. H.; Slok, F. A.; Teuber, L.; Demnitz, J. US 7429618, 2008.
|
|
|
(f) Langler, R. F.; Paddock, R. L.; Thompson, D. B.; Crandall, I.; Ciach, M.; Kain, K. C. Aust. J. Chem. 2003, 56, 1127.
doi: 10.1071/CH03073 |
|
|
(g) Ito, N.; Kurimura, M.; Yamauchi, T.; Segawa, C.; Sasaki, H.; Tai, K.; Arai, K.; Shinohara, T. WO 2009145357, 2009.
|
|
| [6] |
(a) Turner, A. B. Q. Rev. Chem. Soc. 1964, 18, 347.
|
|
(b) Peter, M. G. Angew. Chem. Int. Ed. 1989, 28, 555.
doi: 10.1002/(ISSN)1521-3773 |
|
|
(c) Huang, X. Y.; Ding, R.; Mo, Z. Y.; Xu, Y. L.; Tang, H. T.; Wang, H. S.; Chen, Y. Y.; Pan, Y. M. Org. Lett. 2018, 20, 4819.
doi: 10.1021/acs.orglett.8b01970 |
|
|
(d) Wu, Q. Y.; Ao, G. Z.; Liu, F. Org. Chem. Front. 2018, 5, 2061.
doi: 10.1039/C8QO00428E |
|
|
(e) Ke, M.; Song, Q. Adv. Synth. Catal. 2017, 359, 384.
doi: 10.1002/adsc.201600991 |
|
|
(f) Li, S.; Liu, Y.; Huang, B.; Zhou, T.; Tao, H.; Xiao, Y.; Liu, L.; Zhang, J. ACS Catal. 2017, 7, 2805.
doi: 10.1021/acscatal.7b00030 |
|
|
(g) Huang, G. B.; Huang, W. H.; Guo, J.; Xu, D. L.; Qu, X. C.; Zhai, P. H.; Zheng, X. H.; Weng, J.; Lu, G. Adv. Synth. Catal. 2019, 361, 1241.
doi: 10.1002/adsc.v361.6 |
|
|
(h) Chu, W. D.; Zhang, L. F.; Bao, X.; Zhao, X. H.; Zeng, C.; Du, J. Y.; Zhang, G. B.; Wang, F. X.; Ma, X. Y.; Fan, C. A. Angew. Chem. Int. Ed. 2013, 52, 9229.
doi: 10.1002/anie.201303928 |
|
|
(i) Lou, Y.; Cao, P.; Jia, T.; Zhang, Y.; Wang, M.; Liao, J. Angew. Chem. Int. Ed. 2015, 54, 12134.
doi: 10.1002/anie.201505926 |
|
|
(j) Wang, J.-Y.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Org. Chem. Front. 2020, 7, 1743.
doi: 10.1039/D0QO00387E |
|
|
(k) Zuo, H.-D.; Hao, W.-J.; Zhu, C.-F.; Guo, C.; Tu, S.-J.; Jiang, B. Org. Lett. 2020, 22, 4471.
doi: 10.1021/acs.orglett.0c01470 |
|
|
(l) Chen, K.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Green Chem. 2019, 21, 675.
doi: 10.1039/c8gc03593h |
|
| [7] |
(a) Alejandro, P.; Mariola, T. ChemCatChem 2015, 7, 1524.
doi: 10.1002/cctc.v7.10 pmid: 30203649 |
|
(b) Gai, K.; Fang, X.; Li, X.; Xu, J.; Wu, X.; Lin, A.; Yao, H. Chem. Commun. 2015, 51, 15831.
doi: 10.1039/C5CC06287J pmid: 30203649 |
|
|
(c) Goswami, P.; Singh, G.; Anand, R. V. Org. Lett. 2017, 19, 1982.
doi: 10.1021/acs.orglett.7b00508 pmid: 30203649 |
|
|
(d) Liao, J. Y.; Ni, Q.; Zhao, Y. Org. Lett. 2017, 19, 4074.
doi: 10.1021/acs.orglett.7b01851 pmid: 30203649 |
|
|
(e) Shirsath, S. R.; Shinde, G. H.; Shaikh, A. C.; Muthukrishnan, M. J. Org. Chem. 2018, 83, 12305.
doi: 10.1021/acs.joc.8b01926 pmid: 30203649 |
|
|
(f) Roy, D.; Panda, G. Synthesis 2019, 51, 4434.
doi: 10.1055/s-0039-1690677 pmid: 30203649 |
|
|
(g) Terashima, K.; Kawasaki-Takasuka, T.; Agou, T.; Kubota, T.; Yamazaki, T. Chem. Commun. 2020, 56, 3031.
doi: 10.1039/C9CC08936E pmid: 30203649 |
|
|
(h) Xiu, H.; Li, T.; Song, C.; Ma, Y. Eur. J. Org. Chem. 2020, 6068.
pmid: 30203649 |
|
|
(i) Wang, Z.; Zhu, Y.; Pan, X.; Wang, G.; Liu, L. Angew. Chem. Int. Ed. 2020, 59, 3053.
doi: 10.1002/anie.v59.8 pmid: 30203649 |
|
|
(j) Pan, X.; Wang, X.; Kan, L.; Mao, Y.; Zhu, Y.; Liu, L. Chem. Sci. 2020, 11, 2414.
doi: 10.1039/C9SC05894J pmid: 30203649 |
|
|
(k) Zhang, S.; Zhao, Y.; Li, Q.; Zhang, J.; Hou, Z.; Liu, Y.; Yu, Y.; Peng, D.; Wang, F.; Li, B.; Li, J. Chin. J. Org. Chem. 2019, 39, 709. (in Chinese)
doi: 10.6023/cjoc201808045 pmid: 30203649 |
|
|
(张硕, 赵宁, 李庆刚, 张嘉祺, 侯梓桐, 刘一帆, 于一涛, 彭丹, 王峰, 李冰, 李金辉, 有机化学, 2019, 39, 709.)
doi: 10.6023/cjoc201808045 pmid: 30203649 |
|
|
(l) Zhang, S.; Peng, D.; Zhao, Y.; Yu, Y.; Wang, F.; Liu, H.; Yi, G. Chin. J. Org. Chem. 2019, 39, 555. (in Chinese)
doi: 10.6023/cjoc201807017 pmid: 30203649 |
|
|
(张硕, 彭丹, 赵宁, 于一涛, 王峰, 刘海龙, 伊港, 有机化学, 2019, 39, 555.)
doi: 10.6023/cjoc201807017 pmid: 30203649 |
|
| [8] |
(m) Liu, L.; Zhang, J.-L. Chin. J. Org. Chem. 2019, 39, 3308. (in Chinese)
doi: 10.6023/cjoc201900004 |
|
(刘路, 张俊良, 有机化学, 2019, 39, 3308.)
doi: 10.6023/cjoc201900004 |
|
| [9] |
(a) Guan, X.-Y.; Zhang, L.-D.; You, P.-S.; Liu, S.-S.; Liu, Z.-Q. Tetrahedron Lett. 2019, 60, 244.
doi: 10.1016/j.tetlet.2018.12.023 |
|
(b) Das, D.; Ghosh, K. G.; Chandu, P.; Sureshkumar, D. J. Org. Chem. 2020, 85, 14201.
doi: 10.1021/acs.joc.0c01752 |
|
|
(c) Liu, T.; Liu, J.; Xia, S.; Meng, J.; Shen, X.; Zhu, X.; Chen, W.; Sun, C.; Cheng, F. ACS Omega 2018, 3, 1409.
doi: 10.1021/acsomega.7b01745 |
|
|
(d) Dong, N.; Zhang, Z.-P.; Xue, X.-S.; Li, Xin.; Cheng, J.-P. Angew. Chem. Int. Ed. 2016, 55, 1460.
doi: 10.1002/anie.201509110 |
|
|
(e) Jadhav, A. S.; Anand, R. V. Eur. J. Org. Chem. 2017, 3716.
|
|
|
(f) Liang, X.; Xu, H.; Li, H.; Chen, L.; Lu, H. Eur. J. Org. Chem. 2020, 217.
|
|
|
(g) Dubeya, A.; Manda, P. K. Synlett. 2020, 31, 1713.
doi: 10.1055/s-0040-1707189 |
|
| [10] |
(a) Ma, Y.; Pang, J.; Pan, X.; Ma, S.; Liu, X.; Liu, L. Synlett 2020, 31, 1619.
doi: 10.1055/s-0040-1706408 |
|
(b) Qi, Y.; Zhang, F.; Wang, L.; Feng, A.; Zhu, R.; Sun, S. Li, W.; Liu, L. Org. Biomol. Chem. 2020, 18, 3522.
doi: 10.1039/D0OB00551G |
|
|
(c) Pan, X; Cao, M.; Li, S.; Wang, H.; Liu, X.; Liu, L. Eur. J. Org. Chem. 2021, 1643.
|
|
|
(d) Wang, L.; Wang, N.; Qi, Y.; Liu, X.; Li, W.; Liu, L. Chin. J. Org. Chem. 2020, 40, 3934. (in Chinese)
doi: 10.6023/cjoc202004027 |
|
|
(王琳, 王楠, 齐越, 孙书涛, 刘希功, 李伟, 刘磊, 有机化学, 2020, 40, 3934.)
doi: 10.6023/cjoc202004027 |
|
| [11] |
(a) Petrov, V. A. Fluorinated Heterocyclic Compounds: Synthesis Chemistry, and Applications, Wiley, Hoboken, 2009.
pmid: 25474722 |
|
(b) Nie, J.; Guo, H.-C.; Cahard, D.; Ma, J.-A. Chem. Rev. 2011, 111, 455.
doi: 10.1021/cr100166a pmid: 25474722 |
|
|
(c) Charpentier, J.; Früh, N.; Togni, A. Chem. Rev. 2015, 115, 650.
doi: 10.1021/cr500223h pmid: 25474722 |
|
|
(d) Liu, X.; Xu, C.; Wang, M.; Liu, Q. Chem. Rev. 2015, 115, 683.
doi: 10.1021/cr400473a pmid: 25474722 |
|
|
(e) Campbell, M. G.; Ritter, T. Chem. Rev. 2015, 115, 612.
doi: 10.1021/cr500366b pmid: 25474722 |
|
|
(f) Ni, C.; Hu, M.; Hu, J. Chem. Rev. 2015, 115, 765.
doi: 10.1021/cr5002386 pmid: 25474722 |
|
|
(g) Wang, Z.; Mao, Y.; Guan, H.; Cao, M.; Hua, J.; Feng, L.; Liu, L. Chin. Chem. Lett. 2019, 30, 1241.
doi: 10.1016/j.cclet.2019.03.019 pmid: 25474722 |
|
|
(h) Zhao, R.; Feng, G.; Xin, X.; Guan, H.; Hua, J.; Wan, R.; Li, W.; Liu, L. Chin. Chem. Lett. 2019, 30, 1432.
doi: 10.1016/j.cclet.2019.03.027 pmid: 25474722 |
|
| [12] |
Jiang, C.; Chen, Y.; Huang, G.; Ni, C.; Liu, X.; Lu, H. Asian J. Org. Chem. 2019, 8, 257.
doi: 10.1002/ajoc.v8.2 |
| [1] | 夏登鹏, 罗锦昀, 何林, 蔡志华, 杜广芬. 氮杂环卡宾催化的五氟苯基硫醚的合成[J]. 有机化学, 2024, 44(2): 622-630. |
| [2] | 梅青刚, 李清寒. 可见光促进C(3)(杂)芳硫基吲哚化合物的合成研究进展[J]. 有机化学, 2024, 44(2): 398-408. |
| [3] | 贾小英, 普佳霞, 韩丽荣, 李清寒. 含双杂原子苯并[d]五元杂环硫醚类化合物的合成研究进展[J]. 有机化学, 2024, 44(1): 18-40. |
| [4] | 王灵娜, 刘晓庆, 林钢, 金泓颖, 焦民均, 刘雪粉, 罗书平. 光促进双(4-二苯甲酮)苯醚催化C(sp3)—H键活化构建C—S键[J]. 有机化学, 2023, 43(8): 2848-2854. |
| [5] | 马献涛, 闫晓雨, 朱影影, 牛双林, 王喻璇, 袁超. 水促进下杂芳基硫醚的绿色合成[J]. 有机化学, 2023, 43(6): 2136-2142. |
| [6] | 童宇星, 王子维, 刘奔, 徐耀威, 高颂, 唐向兵, 张兴华. 吲哚-3-硫醚类化合物的合成研究进展[J]. 有机化学, 2023, 43(4): 1310-1324. |
| [7] | 程飞, 孙琪雯, 卢江溶, 王兴兰, 张吉泉. 芳基二硫醚作为自由基硫试剂构建C—S键研究进展[J]. 有机化学, 2023, 43(11): 3728-3744. |
| [8] | 周怡, 李卓骏, 胡明辉, 严兆华, 林森. SO2F2/H2O2/碱和硫醚的氧化反应[J]. 有机化学, 2022, 42(5): 1545-1550. |
| [9] | 吴逾诸, 申盼盼, 段文增, 马玉道. 卡宾催化对亚甲基苯醌的不对称硼化反应的研究[J]. 有机化学, 2022, 42(5): 1483-1492. |
| [10] | 王秀, 段文贵, 林桂汕, 李宝谕, 张文静, 雷福厚. 含天然蒎烯结构的4-酰基-3-氨基-1,2,4-三唑-硫醚衍生物的合成、抑菌活性、三维定量构效关系及分子对接研究[J]. 有机化学, 2022, 42(3): 871-883. |
| [11] | 吴豪志, 罗田, 姜建文, 万结平. 碘化钾催化无保护8-氨基喹啉的选择性C(5)-芳基硫醚化和C(5),C(7)-双芳基硫醚化及吲哚C(2),C(3)-双芳基硫醚化反应[J]. 有机化学, 2022, 42(11): 3721-3729. |
| [12] | 朱海梦, 王超, 宗利利. 亚砜化合物的生物活性研究和不对称合成进展[J]. 有机化学, 2021, 41(9): 3431-3447. |
| [13] | 王东琳, 阚玲珑, 马玉道, 刘磊. 叔丁醇钠催化的δ-腈基-δ-芳基-双取代的对亚甲基苯醌和二芳基氧磷的磷氢化反应研究[J]. 有机化学, 2021, 41(8): 3192-3203. |
| [14] | 王兴越, 许昌林, 关宏宇, 林觅, 黄鹏. 无外加溶剂条件下二甲氨基硫代甲酰氯对亚砜的脱氧还原[J]. 有机化学, 2021, 41(8): 3330-3334. |
| [15] | 李成飞, 岑波, 段文贵, 林桂汕, 王秀, 李宝谕. 含天然苯乙烯结构的4-甲基-1,2,4-三唑-硫醚化合物的合成、除草活性及三维定量构效关系(3D-QSAR)研究[J]. 有机化学, 2021, 41(6): 2485-2495. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||