有机化学 ›› 2022, Vol. 42 ›› Issue (8): 2574-2581.DOI: 10.6023/cjoc202203019 上一篇 下一篇
研究论文
收稿日期:2022-03-08
修回日期:2022-04-11
发布日期:2022-04-22
通讯作者:
张志鹏
基金资助:Received:2022-03-08
Revised:2022-04-11
Published:2022-04-22
Contact:
Zhipeng Zhang
Supported by:文章分享

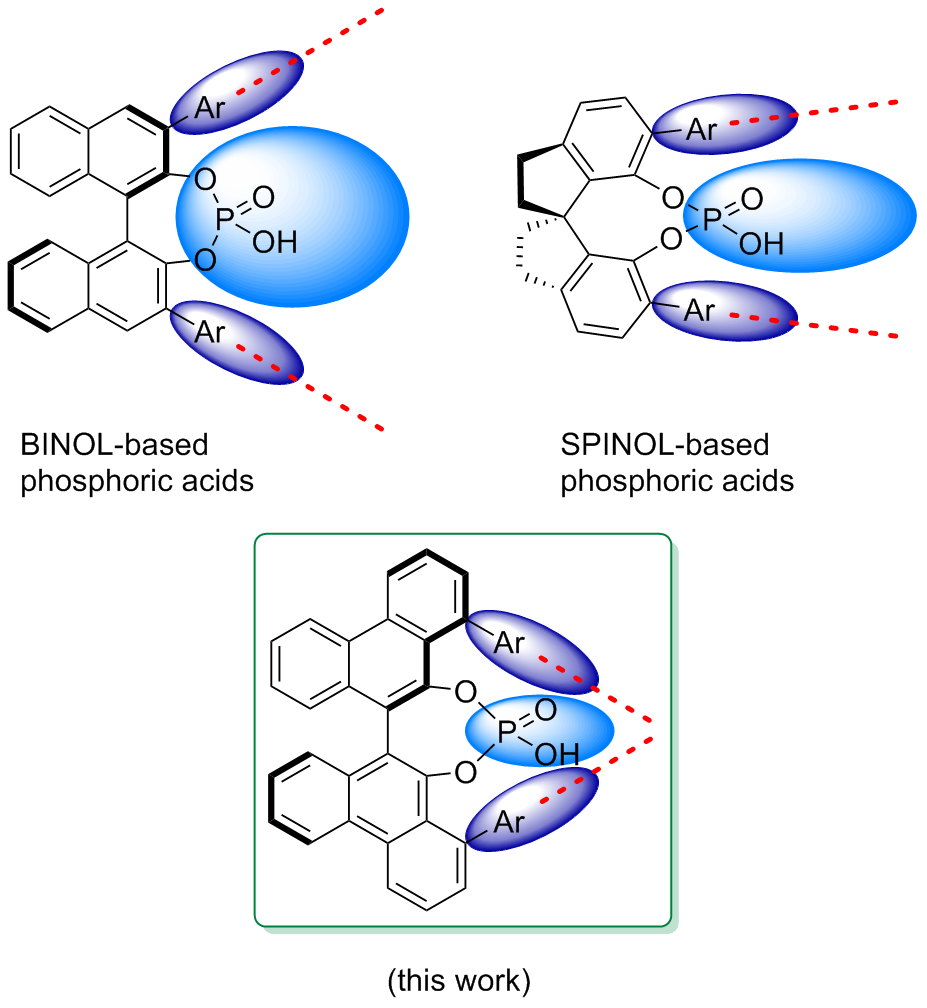
以轴手性的1,1'-联-2-萘酚(BINOL)和1,1'-螺二氢茚-7,7'-二酚(SPINOL)为骨架的手性磷酸已经被广泛应用于不对称催化领域, 在数以百计的反应中表现出了优秀的对映选择性. 然而在位阻较小且不含芳环的底物参与的不对称催化反应中, 这两类手性磷酸所表现出的对映选择性通常不理想, 主要原因在于BINOL的3,3'位取代基和SPINOL的6,6'位取代基向外侧扩展的固有特性使得催化活性位点附近的手性环境相对宽松. 为了发展适用于这类底物的手性磷酸催化剂, 设计了一种以9,9'-联-10-菲酚(BIFOL)为骨架的1,1'位具有向内侧伸展的芳基取代基的具有紧密手性环境的磷酸, 以廉价易得的间二溴苯为起始原料, 首先合成关键中间体8-溴-9-菲酚, 再通过氧化偶联合成1,1'位溴代的BIFOL, 然后利用Suzuki-Miyaura偶联反应引入芳基取代基, 最后将芳基取代的BIFOL与三氯氧磷反应的产物磷酰氯水解, 经过十二步反应合成了目标分子, 并且通过单晶X射线衍射分析确定了其结构.
岑守义, 张志鹏. 具有紧密手性环境的联菲酚骨架磷酸的合成[J]. 有机化学, 2022, 42(8): 2574-2581.
Shouyi Cen, Zhipeng Zhang. Synthesis of Biphenanthrol-Based Confined Chiral Phosphoric Acid[J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2574-2581.

| [1] |
(a) Akiyama, T.; Itoh, J.; Yokota, K.; Fuchibe, K. Angew. Chem., Int. Ed. 2004, 43, 1566.
doi: 10.1002/anie.200353240 pmid: 15113196 |
|
(b) Uraguchi, D.; Terada, M. J. Am. Chem. Soc. 2004, 126, 5356.
pmid: 15113196 |
|
| [2] |
(a) Akiyama, T.; Mori, K. Chem. Rev. 2015, 115, 9277.
doi: 10.1021/acs.chemrev.5b00041 pmid: 22282764 |
|
(b) Parmar, D.; Sugiono, E.; Raja, S.; Rueping, M. Chem. Rev. 2014, 114, 9047.
doi: 10.1021/cr5001496 pmid: 22282764 |
|
|
(c) Xu, B.; Zhu, S.-F.; Xie, X.-L.; Shen, J.-J.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2011, 50, 11483.
doi: 10.1002/anie.201105485 pmid: 22282764 |
|
|
(d) Sedgwick, D. M.; Grayson, M. N.; Fustero, S.; Barrio, P. Synthesis 2018, 50, 1935.
doi: 10.1055/s-0036-1589532 pmid: 22282764 |
|
|
(e) Cheng, J. K.; Xiang, S.-H.; Li, S.; Ye, L.; Tan, B. Chem. Rev. 2021, 121, 4805.
doi: 10.1021/acs.chemrev.0c01306 pmid: 22282764 |
|
|
(f) Zheng, C.; You, S.-L. Chem. Soc. Rev. 2012, 41, 2498.
doi: 10.1039/c1cs15268h pmid: 22282764 |
|
|
(g) Li, S.; Xiang, S.-H.; Tan, B. Chin. J. Chem. 2020, 38, 213.
pmid: 22282764 |
|
|
(h) Lin, X.; Wang, L.; Han, Z.; Chen, Z. Chin. J. Chem. 2021, 39, 802.
pmid: 22282764 |
|
|
(i) Zhou, X.-L.; Su, Y.-L.; Wang, P.-S.; Gong, L.-Z. Acta Chim. Sinica 2018, 76, 857. (in Chinese)
pmid: 22282764 |
|
|
(周霄乐, 苏永亮, 汪普生, 龚流柱, 化学学报, 2018, 76, 857.)
doi: 10.6023/A18060235 pmid: 22282764 |
|
|
(j) Sheng, F.-T.; Li, Z.-M.; Zhang, Y.-Z.; Sun, L.-X.; Zhang, Y.-C.; Tan, W.; Shi, F. Chin. J. Chem. 2020, 38, 583.
doi: 10.1002/cjoc.202000022 pmid: 22282764 |
|
|
(k) Mo, N.-F.; Yu, L.; Zhang, Y.; Yao, Y.-H.; Kou, X.; Ren, Z.-H.; Guan, Z.-H. CCS Chem. 2020, 2, 1775.
pmid: 22282764 |
|
|
(l) Lv, X.; Liu, S.; Zhou, S.; Dong, G.; Xing, D.; Xu, X.; Hu, W. CCS Chem. 2020, 2, 1903.
pmid: 22282764 |
|
| [3] |
(a) Yang, L.-L.; Ouyang, J.; Zou, H.-N.; Zhu, S.-F.; Zhou, Q.-L. J. Am. Chem. Soc. 2021, 143, 6401.
doi: 10.1021/jacs.1c03435 |
|
(b) Yang, L.-L.; Evans, D.; Xu, B.; Li, W.-T.; Li, M.-L.; Zhu, S.-F.; Houk, K. N.; Zhou, Q.-L. J. Am. Chem. Soc. 2020, 142, 12394.
doi: 10.1021/jacs.0c04725 |
|
|
(c) Li, Y.; Zhao, Y.-T.; Zhou, T.; Chen, M.-Q.; Li, Y.-P.; Huang, M.-Y.; Xu, Z.-C.; Zhu, S.-F.; Zhou, Q.-L. J. Am. Chem. Soc. 2020, 142, 10557.
doi: 10.1021/jacs.0c04532 |
|
|
(d) Li, M.; Chen, M.; Xu, B.; Zhu, S.; Zhou, Q. Acta Chim. Sinica 2018, 76, 883. (in Chinese)
doi: 10.6023/A18060234 |
|
|
(李茂霖, 陈梦青, 徐彬, 朱守非, 周其林, 化学学报, 2018, 76, 883.)
doi: 10.6023/A18060234 |
|
|
(e) Ren, Y.-Y.; Zhu, S.-F.; Zhou, Q.-L. Org. Biomol. Chem. 2018, 16, 3087.
doi: 10.1039/C8OB00473K |
|
|
(f) Guo, J.-X.; Zhou, T.; Xu, B.; Zhu, S.-F.; Zhou, Q.-L. Chem. Sci. 2016, 7, 1104.
doi: 10.1039/C5SC03558A |
|
|
(g) Xu, B.; Li, M.-L.; Zuo, X.-D.; Zhu, S.-F.; Zhou, Q.-L. J. Am. Chem. Soc. 2015, 137, 8700.
doi: 10.1021/jacs.5b05086 |
|
| [4] |
(a) Xia, Z.-L.; Xu-Xu, Q.-F.; Zheng, C.; You, S.-L. Chem. Soc. Rev. 2020, 49, 286.
doi: 10.1039/C8CS00436F |
|
(b) Cai, Q.; Liu, C.; Liang, X.-W.; You, S.-L. Org. Lett. 2012, 14, 4588.
doi: 10.1021/ol302043s |
|
|
(c) Zhou, Y.; Xia, Z.-L.; Gu, Q.; You, S.-L. Org. Lett. 2017, 19, 762.
doi: 10.1021/acs.orglett.6b03610 |
|
|
(d) Xia, Z.-L.; Zheng, C.; Wang, S.-G.; You, S.-L. Angew. Chem., Int. Ed. 2018, 57, 2653.
doi: 10.1002/anie.201712435 |
|
|
(e) Xia, Z.-L.; Zheng, C.; Xu, R.-Q.; You, S.-L. Nat. Commun. 2019, 10, 3150.
doi: 10.1038/s41467-019-11109-9 |
|
|
(f) Duan, D.; Yin, Q.; Wang, S.; Gu, Q.; You, S. Acta Chim. Sinica 2014, 72, 1001. (in Chinese)
doi: 10.6023/A14060497 |
|
|
(段德河, 殷勤, 王守国, 顾庆, 游书力, 化学学报, 2014, 72, 1001.)
doi: 10.6023/A14060497 |
|
| [5] |
(a) Zhang, J.; Yu, P.; Li, S.-Y.; Sun, H.; Xiang, S.-H.; Wang, J.; Houk, K. N.; Tan, B. Science 2018, 361, 1087.
|
|
(b) Yang, J.; Zhang, J.-W.; Bao, W.; Qiu, S.-Q.; Li, S.; Xiang, S.-H.; Song, J.; Zhang, J.; Tan, B. J. Am. Chem. Soc. 2021, 143, 12924.
doi: 10.1021/jacs.1c05079 |
|
|
(c) An, Q.-J.; Xia, W.; Ding, W.-Y.; Liu, H.-H.; Xiang, S.-H.; Wang, Y.-B.; Zhong, G.; Tan, B. Angew. Chem., Int. Ed. 2021, 60, 24888.
doi: 10.1002/anie.202111251 |
|
|
(d) Mao, J.-H.; Wang, Y.-B.; Yang, L.; Xiang, S.-H.; Wu, Q.-H.; Cui, Y.; Lu, Q.; Lv, J.; Li, S.; Tan, B. Nat. Chem. 2021, 13, 982.
doi: 10.1038/s41557-021-00750-x |
|
|
(e) Zhang, L.; Shen, J.; Wu, S.; Zhong, G.; Wang, Y.-B.; Tan, B. Angew. Chem., Int. Ed. 2020, 59, 23077.
doi: 10.1002/anie.202010598 |
|
|
(f) Xia, W.; An, Q.-J.; Xiang, S.-H.; Li, S.; Wang, Y.-B.; Tan, B. Angew. Chem., Int. Ed. 2020, 59, 6775.
doi: 10.1002/anie.202000585 |
|
|
(g) Wang, Y.-B.; Yu, P.; Zhou, Z.-P.; Zhang, J.; Wang, J.; Luo, S.-H.; Gu, Q.-S.; Houk, K. N.; Tan, B. Nat. Catal. 2019, 2, 504.
doi: 10.1038/s41929-019-0278-7 |
|
|
(h) Da, B.-C.; Xiang, S.-H.; Li, S.; Tan, B. Chin. J. Chem. 2021, 39, 1787.
doi: 10.1002/cjoc.202000751 |
|
| [6] |
Li, S.; Zhang, J.-W.; Li, X.-L.; Cheng, D.-J.; Tan, B. J. Am. Chem. Soc. 2016, 138, 16561.
doi: 10.1021/jacs.6b11435 |
| [7] |
(a) Qian, D.; Chen, M.; Bissember, A. C.; Sun, J. Angew. Chem., Int. Ed. 2018, 57, 3763.
doi: 10.1002/anie.201712395 pmid: 33382271 |
|
(b) Sun, S.; Wang, Z.; Li, S.; Zhou, C.; Song, L.; Huang, H.; Sun, J. Org. Lett. 2021, 23, 554.
doi: 10.1021/acs.orglett.0c04074 pmid: 33382271 |
|
| [8] |
Zhang, R.; Ge, S.; Sun, J. J. Am. Chem. Soc. 2021, 143, 12445.
doi: 10.1021/jacs.1c05709 |
| [9] |
(a) Li, Z.-L.; Fang, G.-C.; Gu, Q.-S.; Liu, X.-Y. Chem. Soc. Rev. 2020, 49, 32.
doi: 10.1039/C9CS00681H |
|
(b) Zeng, Y.; Liu, X.-D.; Guo, X.-Q.; Gu, Q.-S.; Li, Z.-L.; Chang, X.-Y.; Liu, X.-Y. Sci. China Chem. 2019, 62, 1529.
doi: 10.1007/s11426-019-9528-2 |
|
|
(c) Lin, J.-S.; Li, T.-T.; Lin, J.-R.; Jiao, G.-Y.; Gu, Q.-S.; Cheng, J.-T.; Guo, Y.-L.; Hong, X.; Liu, X.-Y. J. Am. Chem. Soc. 2019, 141, 1074.
doi: 10.1021/jacs.8b11736 |
|
|
(d) Lin, J.-S.; Wang, F.-L.; Dong, X.-Y.; He, W.-W.; Yuan, Y.; Chen, S.; Liu, X.-Y. Nat. Commun. 2017, 8, 14841.
doi: 10.1038/ncomms14841 |
|
|
(e) Cheng, Y.-F.; Dong, X.-Y.; Gu, Q.-S.; Yu, Z.-L.; Liu, X.-Y. Angew. Chem., Int. Ed. 2017, 56, 8883.
doi: 10.1002/anie.201702925 |
|
|
(f) Lin, J.-S.; Dong, X.-Y.; Li, T.-T.; Jiang, N.-C.; Tan, B.; Liu, X.-Y. J. Am. Chem. Soc. 2016, 138, 9357.
doi: 10.1021/jacs.6b04077 |
|
|
(g) Yu, P.; Lin, J.-S.; Li, L.; Zheng, S.-C.; Xiong, Y.-P.; Zhao, L.-J.; Tan, B.; Liu, X.-Y. Angew. Chem., Int. Ed. 2014, 53, 11890.
doi: 10.1002/anie.201405401 |
|
|
(h) Wang, Z.; Cheng, J.-T.; Shi, Z.; Wang, N.; Zhan, F.; Jiang, S.-P.; Lin, J.-S.; Jiang, Y.; Liu, X.-Y. ChemCatChem 2021, 13, 185.
doi: 10.1002/cctc.202001398 |
|
|
(i) Li, X.-F.; Lin, J.-S.; Wang, J.; Li, Z.-L.; Gu, Q.-S.; Liu, X.-Y. Acta Chim. Sinica 2018, 76, 878. (in Chinese)
doi: 10.6023/A18100413 |
|
|
(李雪飞, 林进顺, 王建, 李忠良, 顾强帅, 刘心元, 化学学报, 2018, 76, 878.)
doi: 10.6023/A18100413 |
|
|
(j) Wang, F.-L.; Dong, X.-Y.; Lin, J.-S.; Zeng, Y.; Jiao, G.-Y.; Gu, Q.-S.; Guo, X.-Q.; Ma, C.-L.; Liu, X.-Y. Chem 2017, 3, 979.
doi: 10.1016/j.chempr.2017.10.008 |
|
| [10] |
(a) Liao, G.; Zhang, T.; Jin, L.; Wang, B.-J.; Xu, C.-K.; Lan, Y.; Zhao, Y.; Shi, B.-F. Angew. Chem., Int. Ed. 2022, 61, e202115221.
|
|
(b) Jin, L.; Zhang, P.; Li, Y.; Yu, X.; Shi, B.-F. J. Am. Chem. Soc. 2021, 143, 12335.
doi: 10.1021/jacs.1c06236 |
|
|
(c) Zhan, B.-B.; Jia, Z.-S.; Luo, J.; Jin, L.; Lin, X.-F.; Shi, B.-F. Org. Lett. 2020, 22, 9693.
doi: 10.1021/acs.orglett.0c03757 |
|
|
(d) Zhan, B.-B.; Wang, L.; Luo, J.; Lin, X.-F.; Shi, B.-F. Angew. Chem., Int. Ed. 2020, 59, 3568.
doi: 10.1002/anie.201915674 |
|
|
(e) Luo, J.; Zhang, T.; Wang, L.; Liao, G.; Yao, Q.-J.; Wu, Y.-J.; Zhan, B.-B.; Lan, Y.; Lin, X.-F.; Shi, B.-F. Angew. Chem., Int. Ed. 2019, 58, 6708.
doi: 10.1002/anie.201902126 |
|
| [11] |
(a) Guo, Z.; Xie, J.; Hu, T.; Chen, Y.; Tao, H.; Yang, X. Chem. Commun. 2021, 57, 9394.
doi: 10.1039/D1CC03117A |
|
(b) Tang, M.; Gu, H.; He, S.; Rajkumar, S.; Yang, X. Angew. Chem., Int. Ed. 2021, 60, 21334.
doi: 10.1002/anie.202106151 |
|
|
(c) Pan, Y.; Wang, D.; Chen, Y.; Zhang, D.; Liu, W.; Yang, X. ACS Catal. 2021, 11, 8443.
doi: 10.1021/acscatal.1c02331 |
|
|
(d) Chen, Y.; Zhu, C.; Guo, Z.; Liu, W.; Yang, X. Angew. Chem., Int. Ed. 2021, 60, 5268.
doi: 10.1002/anie.202015008 |
|
|
(e) Pan, Y.; Jiang, Q.; Rajkumar, S.; Zhu, C.; Xie, J.; Yu, S.; Chen, Y.; He, Y.-P.; Yang, X. Adv. Syn. Catal. 2021, 363, 200.
doi: 10.1002/adsc.202001051 |
|
|
(f) Liu, W.; Jiang, Q.; Yang, X. Angew. Chem., Int. Ed. 2020, 59, 23598.
doi: 10.1002/anie.202009395 |
|
|
(g) He, F.; Shen, G.; Yang, X. Chin. J. Chem. 2022, 40, 15.
doi: 10.1002/cjoc.202100514 |
|
| [12] |
(a) Zhang, Y.-C.; Jiang, F.; Shi, F. Acc. Chem. Res. 2020, 53, 425.
doi: 10.1021/acs.accounts.9b00549 |
|
(b) Li, T.-Z.; Liu, S.-J.; Sun, Y.-W.; Deng, S.; Tan, W.; Jiao, Y.; Zhang, Y.-C.; Shi, F. Angew. Chem., Int. Ed. 2021, 60, 2355.
doi: 10.1002/anie.202011267 |
|
|
(c) Ma, C.; Jiang, F.; Sheng, F.-T.; Jiao, Y.; Mei, G.-J.; Shi, F. Angew. Chem., Int. Ed. 2019, 58, 3014.
doi: 10.1002/anie.201811177 |
|
|
(d) Tan, W.; Li, X.; Gong, Y.-X.; Ge, M.-D.; Shi, F. Chem. Commun. 2014, 50, 15901.
doi: 10.1039/C4CC07246D |
|
|
(e) Shi, F.; Zhang, H.-H.; Sun, X.-X.; Liang, J.; Fan, T.; Tu, S.-J. Chem. Eur. J. 2015, 21, 3465.
doi: 10.1002/chem.201405245 |
|
|
(f) Zhao, J.-J.; Sun, S.-B.; He, S.-H.; Wu, Q.; Shi, F. Angew. Chem., Int. Ed. 2015, 54, 5460.
doi: 10.1002/anie.201500215 |
|
|
(g) Wang, J.-Y.; Sun, M.; Yu, X.-Y.; Zhang, Y.-C.; Tan, W.; Shi, F. Chin. J. Chem. 2021, 39, 2163.
doi: 10.1002/cjoc.202100214 |
|
| [13] |
(a) Birman, V. B.; Rheingold, A. L.; Lam, K.-C. Tetrahedron: Asymmetry 1999, 10, 125.
|
|
(b) Zhang, J.-H.; Liao, J.; Cui, X.; Yu, K.-B.; Zhu, J.; Deng, J.-G.; Zhu, S.-F.; Wang, L.-X.; Zhou, Q.-L.; Chung, L. W.; Ye, T. Tetrahedron: Asymmetry 2002, 13, 1363.
|
|
|
(c) Brunel, J. M. Chem. Rev. 2005, 105, 857.
doi: 10.1021/cr040079g |
|
| [14] |
(a) Cram, D. J.; Helgeson, R. C.; Peacock, S. C.; Kaplan, L. J.; Domeier, L. A.; Moreau, P.; Koga, K.; Mayer, J. M.; Chao, Y.; Siegel, M. G.; Hoffman, D. H.; Sogah, G. D. Y. J. Org. Chem. 1978, 43, 1930.
doi: 10.1021/jo00404a019 |
|
(b) Chen, X.-H.; Xu, X.-Y.; Liu, H.; Cun, L.-F.; Gong, L.-Z. J. Am. Chem. Soc. 2006, 128, 14802.
doi: 10.1021/ja065267y |
|
|
(c) Furuno, H.; Kambara, T.; Tanaka, Y.; Hanamoto, T.; Kagawa, T.; Inanaga, J. Tetrahedron Lett. 2003, 44, 6129.
doi: 10.1016/S0040-4039(03)01460-6 |
|
|
(d) Shigeyoshi, K.; Nobuyuki, T.; Masatoshi, M.; Hiroshi, S. Bull. Chem. Soc. Jpn. 1987, 60, 2307.
doi: 10.1246/bcsj.60.2307 |
|
|
(e) Frankland, P. F.; Twiss, D. F. J. Chem. Soc. Trans. 1904, 85, 1666.
doi: 10.1039/CT9048501666 |
|
|
(f) Akiyama, T.; Saitoh, Y.; Morita, H.; Fuchibe, K. Adv. Synth. Catal. 2005, 347, 1523.
doi: 10.1002/adsc.200505167 |
|
| [15] |
(a) Desai, A. A.; Wulff, W. D. Synthesis 2010, 3670.
|
|
(b) Bao, J.; Wulff, W. D.; Rheingold, A. L. J. Am. Chem. Soc. 1993, 115, 3814.
doi: 10.1021/ja00062a073 |
|
|
(c) Bao, J.; Wulff, W. D.; Dominy, J. B.; Fumo, M. J.; Grant, E. B.; Rob, A. C.; Whitcomb, M. C.; Yeung, S. M.; Ostrander, R. L.; Rheingold, A. L. J. Am. Chem. Soc. 1996, 118, 3392.
doi: 10.1021/ja952018t |
|
|
(d) Hu, G.; Holmes, D.; Gendhar, B. F.; Wulff, W. D. J. Am. Chem. Soc. 2009, 131, 14355.
doi: 10.1021/ja903820m |
|
|
(e) Ding, Z.; Osminski, W. E. G.; Ren, H.; Wulff, W. D. Org. Process Res. Dev. 2011, 15, 1089.
doi: 10.1021/op200088b |
|
|
(f) Desai, A. A.; Huang, L.; Wulff, W. D.; Rowland, G. B.; Antilla, J. C. Synthesis 2010, 2106.
|
|
| [16] |
(a) Rowland, G. B.; Zhang, H.; Rowland, E. B.; Chennamadhavuni, S.; Wang, Y.; Antilla, J. C. J. Am. Chem. Soc. 2005, 127, 15696.
pmid: 19905027 |
|
(b) Li, G.; Liang, Y.; Antilla, J. C. J. Am. Chem. Soc. 2007, 129, 5830.
doi: 10.1021/ja070519w pmid: 19905027 |
|
|
(c) Rowland, E. B.; Rowland, G. B.; Rivera-Otero, E.; Antilla, J. C. J. Am. Chem. Soc. 2007, 129, 12084.
pmid: 19905027 |
|
|
(d) Zheng, W.; Zhang, Z.; Kaplan, M. J.; Antilla, J. C. J. Am. Chem. Soc. 2011, 133, 3339.
doi: 10.1021/ja109824x pmid: 19905027 |
|
|
(e) Larson, S. E.; Li, G.; Rowland, G. B.; Junge, D.; Huang, R.; Woodcick, H. L.; Antilla, J. C. Org. Lett. 2011, 13, 2188.
doi: 10.1021/ol200407r pmid: 19905027 |
|
|
(f) Larson, S. E.; Baso, J. C.; Li, G.; Antilla, J. C. Org. Lett. 2009, 11, 5186.
doi: 10.1021/ol902123h pmid: 19905027 |
|
| [17] |
(a) Lee, S.; Kim, S. Tetrahedron Lett. 2009, 50, 3345.
doi: 10.1016/j.tetlet.2009.02.136 |
|
(b) Lu, G.; Birman, V. B. Org. Lett. 2011, 13, 356.
doi: 10.1021/ol102736t |
|
|
(c) Zhuang, M.; Du, H. Org. Biomol. Chem. 2013, 11, 1460.
doi: 10.1039/c3ob27285k |
|
|
(d) Liao, S.; Čoric, I.; Wang, Q.; List, B. J. Am. Chem. Soc. 2012, 134, 10765.
doi: 10.1021/ja3035637 |
|
|
(e) Zhang, Q.-W.; Fan, C.-A.; Zhang, H.-J.; Tu, Y.-Q.; Zhao, Y.-M.; Gu, P.; Chen, Z.-M. Angew. Chem., Int. Ed. 2009, 48, 8572.
doi: 10.1002/anie.200904565 |
|
|
(f) Huang, D.; Wang, H.; Xue, F.; Guan, H.; Li, L.; Peng, X.; Shi, Y. Org. Lett. 2011, 13, 6350.
doi: 10.1021/ol202527g |
|
|
(g) Terada, M.; Tanaka, H.; Sorimachi, K. Synlett 2008, 1661.
|
|
| [18] |
(a) Čorić, I.; List, B. Nature 2012, 483, 315.
doi: 10.1038/nature10932 |
|
(b) Das, S.; Liu, L.; Zheng, Y.; Alachraf, M. W.; Thiel, W.; De, C. K.; List, B. J. Am. Chem. Soc. 2016, 138, 9429.
doi: 10.1021/jacs.6b06626 |
|
|
(c) Liu, L.; Kaib, P. S. J.; Tap, A.; List, B. J. Am. Chem. Soc. 2016, 138, 10822.
doi: 10.1021/jacs.6b07240 |
|
|
(d) Schreyer, L.; Properzi, R.; List, B. Angew. Chem., Int. Ed. 2019, 58, 12761.
doi: 10.1002/anie.201900932 |
|
| [19] |
(a) Grossmann, O.; Maji, R.; Aukland, M. H.; Lee, S.; List, B. Angew. Chem., Int. Ed. 2022, 61, doi: 10.1002/anie.202115036.
doi: 10.1002/anie.202115036 pmid: 30277760 |
|
(b) Díaz-Oviedo, C. D.; Maji, R.; List, B. J. Am. Chem. Soc. 2021, 143, 20598.
doi: 10.1021/jacs.1c10245 pmid: 30277760 |
|
|
(c) Das, S.; Mitschke, B.; De, C. K.; Harden, I.; Bistoni, G.; List, B. Nat. Catal. 2021, 4, 1043.
doi: 10.1038/s41929-021-00714-x pmid: 30277760 |
|
|
(d) Amatov, T.; Tsuji, N.; Maji, R.; Schreyer, L.; Zhou, H.; Leutzsch, M.; List, B. J. Am. Chem. Soc. 2021, 143, 14475.
doi: 10.1021/jacs.1c07447 pmid: 30277760 |
|
|
(e) Ghosh, S.; Das, S.; De, C. K.; Yepes, D.; Neese, F.; Bistoni, G.; Leutzsch, M.; List, B. Angew. Chem., Int. Ed. 2020, 59, 12347.
doi: 10.1002/anie.202000307 pmid: 30277760 |
|
|
(f) Schreyer, L.; Kaib, P. S. J.; Wakchaure, V. N.; Obradors, C.; Properzi, R.; Lee, S.; List, B. Science 2018, 362, 216.
doi: 10.1126/science.aau0817 pmid: 30277760 |
|
|
(g) Gatzenmeier, T.; Turberg, M.; Yepes, D.; Xie, Y.; Neese, F.; Bistoni, G.; List, B. J. Am. Chem. Soc. 2018, 140, 12671.
doi: 10.1021/jacs.8b07092 pmid: 30277760 |
|
| [20] |
(a) Yamamoto, K.; Fukushima, H.; Nakazaki, M. J. Chem. Soc., Chem. Commun. 1984, 1490.
|
|
(b) Yamamoto, K.; Fukushima, H.; Okamoto, Y.; Hatada, K.; Nakazaki, M. J. Chem. Soc., Chem. Commun. 1984, 1111.
|
|
| [21] |
(a) Takizawa, S.; Kodera, J.; Yoshida, Y.; Sako, M.; Breukers, S.; Enders, D.; Sasai, H. Tetrahedron 2014, 70, 1786.
doi: 10.1016/j.tet.2014.01.017 |
|
(b) Xie, S.; Wu, F.; Huang, W. Acta Sci. Nat. Univ. Sunyatseni 1986, 25, 18. (in Chinese)
|
|
|
(谢颂凯, 吴峰, 黄文洪, 中山大学学报(自然科学版), 1986, 25, 18.)
|
|
| [22] |
(a) Takizawa, S.; Katayama, T.; Kameyama, C.; Onitsuka, K.; Suzuki, T.; Yanagida, T.; Kawaia, T.; Sasai, H. Chem. Commun. 2008, 1810.
|
|
(b) Takizawa, S.; Rajesh, D.; Katayama, T.; Sasai, H. Synlett 2009, 1667.
|
|
|
(c) Aydin, J.; Kumar, K. S.; Sayah, M. J.; Wallner, O. A.; Szabó, K. J. J. Org. Chem. 2007, 72, 4689.
doi: 10.1021/jo070288b |
|
| [23] |
Saá, J. M.; Morey, J.; Frontera, A.; Deyá, P. M. J. Am. Chem. Soc. 1995, 117, 1105.
doi: 10.1021/ja00108a029 |
| [24] |
(a) Gualandi, A.; Savoini, A.; Saporetti, R.; Franchi, P.; Lucarini, M.; Cozzi, P. G. Org. Chem. Front. 2018, 5, 1573.
doi: 10.1039/C8QO00061A pmid: 18454526 |
|
(b) Norman, D. W.; Carraz, C. A.; Hyett, D. J.; Pringle, P. G.; Sweeney, J. B.; Orpen, A. G.; Phetmung, H.; Wingad, R. L. J. Am. Chem. Soc. 2008, 130, 6840.
doi: 10.1021/ja800858x pmid: 18454526 |
|
|
(c) Gao, H.; Zhou, Z.; Kwon, D.-H.; Coombs, J.; Jones, S.; Behnke, N. E.; Ess, D. H.; Kürti, L. Nat. Chem. 2017, 9, 681.
doi: 10.1038/nchem.2672 pmid: 18454526 |
|
|
(d) Furusho, Y.; Tsunoda, A.; Aida, T. J. Chem. Soc., Perkin Trans. 1 1996, 183.
pmid: 18454526 |
|
| [25] |
Snieckus, V. Chem. Rev. 1990, 90, 879.
doi: 10.1021/cr00104a001 |
| [26] |
Luliński, S.; Serwatowski, J. J. Org. Chem. 2003, 68, 5384.
doi: 10.1021/jo0340511 |
| [27] |
Tashiro, M.; Nakayama, K. Org. Prep. Proced. Int. 1984, 16, 379.
doi: 10.1080/00304948409457894 |
| [28] |
(a) Eaton, P. E.; Martin, R. M. J. Org. Chem. 1988, 53, 2728.
doi: 10.1021/jo00247a013 |
|
(b) Leggio, A.; Belsito, E. L.; Luca, G. D.; Gioia, M. L. D.; Leotta, V.; Romio, E.; Sicilianoa, C.; Liguori, A. RSC Adv. 2016, 6, 34468.
doi: 10.1039/C5RA24527C |
|
| [29] |
Jørgensen, K. B.; Rantanen, T.; Dörfler, T.; Snieckus, V. J. Org. Chem. 2015, 80, 9410.
doi: 10.1021/acs.joc.5b01300 pmid: 26301487 |
| [30] |
(a) Nakajima, K.; Kojimo, M.; Fujita, J. Chem. Lett. 1986, 15, 1483.
doi: 10.1246/cl.1986.1483 pmid: 11263903 |
|
(b) Hon, S.-W.; Li, C.-H.; Kuo, J.-H.; Barhate, N. B.; Liu, Y.-H.; Wang, Y.; Chen, C.-T. Org. Lett. 2001, 3, 869.
pmid: 11263903 |
|
| [31] |
Lim, J. Y. C.; Marques, I.; Félix, V.; Beer, P. D. J. Am. Chem. Soc. 2017, 139, 12228.
doi: 10.1021/jacs.7b06144 |
| [1] | 向勋, 何照林, 董秀琴. 钯和手性磷酸协同催化高效构建手性分子的研究进展[J]. 有机化学, 2023, 43(3): 791-808. |
| [2] | 陈宇亮, 贺凤开, 王思云, 贾鼎成, 刘亚群, 黄毅勇. 手性磷酸催化α-全碳季碳醛的不对称烯丙基化动力学拆分[J]. 有机化学, 2023, 43(12): 4294-4302. |
| [3] | 周启文, 冯向青, 杨晶, 杜海峰. 基于手性氨硼烷的β-烯胺腈不对称转移氢化反应[J]. 有机化学, 2019, 39(8): 2188-2195. |
| [4] | 张松, 陆俊筑, 叶金星, 段伟良. 手性磷酸控制的不对称碳氢芳基化反应合成平面手性二茂铁化合物[J]. 有机化学, 2016, 36(4): 752-759. |
| [5] | 杜鹏, 周海峰, 沈冠硕, 邹坤. 手性缩醛的催化不对称合成研究进展[J]. 有机化学, 2015, 35(8): 1641-1649. |
| [6] | 苏亚军, 史福强. 手性磷酸在不对称反应中的应用[J]. 有机化学, 2010, 30(04): 486-498. |
| [7] | 高勇军,杨丽华,宋双居,马晶军,唐然肖边瑞环,刘海燕,吴秋华,王春. 手性磷酸催化的有机催化不对称反应[J]. 有机化学, 2008, 28(01): 8-16. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
