Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (6): 1747-1758.DOI: 10.6023/cjoc202112040 Previous Articles Next Articles
ARTICLES
张同利a, 晏君b, 何敬立a, 寇学振b, 申杰峰a, 刘德龙a,*( ), 张万斌a,b,*(
), 张万斌a,b,*( )
)
收稿日期:2021-12-29
修回日期:2022-02-21
发布日期:2022-03-03
通讯作者:
刘德龙, 张万斌
基金资助:
Tongli Zhanga, Jun Yanb, Jingli Hea, Xuezhen Koub, Jiefeng Shena, Delong Liua( ), Wanbin Zhanga,b(
), Wanbin Zhanga,b( )
)
Received:2021-12-29
Revised:2022-02-21
Published:2022-03-03
Contact:
Delong Liu, Wanbin Zhang
Supported by:Share
Tongli Zhang, Jun Yan, Jingli He, Xuezhen Kou, Jiefeng Shen, Delong Liu, Wanbin Zhang. Synthesis of Chiral 5-Aryl-2-oxazolidinones via an Ir-BiphPHOX Catalyzed Enantioselective Hydrogenation[J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1747-1758.
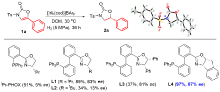
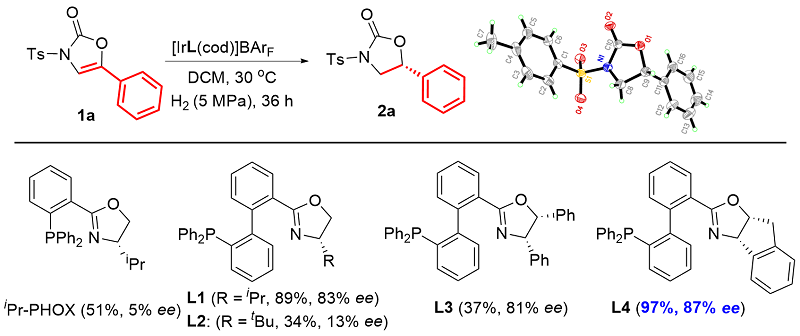
| Entry | Solvent | p(H2)/MPa | Temp./℃ | Conv.b/% | eec/% |
|---|---|---|---|---|---|
| 1 | DCM | 5 | 30 | 97 | 87 |
| 2 | DCE | 5 | 30 | >99 | 93 |
| 3 | PhCl | 5 | 30 | 58 | 93 |
| 4 | Toluene | 5 | 30 | 52 | 69 |
| 5 | o-Xylene | 5 | 30 | 34 | 85 |
| 6 | THF | 5 | 30 | 0 | — |
| 7 | EtOAc | 5 | 30 | 5 | 84 |
| 8 | MeOH | 5 | 30 | 0 | — |
| 9 | DCE | 4 | 30 | 93 | 91 |
| 10 | DCE | 6 | 30 | >99 | 91 |
| 11 | DCE | 5 | 20 | 96 | 90 |
| 12 | DCE | 5 | 50 | >99 | 90 |
| 13d | DCE | 5 | 30 | 82 | 93 |
| Entry | Solvent | p(H2)/MPa | Temp./℃ | Conv.b/% | eec/% |
|---|---|---|---|---|---|
| 1 | DCM | 5 | 30 | 97 | 87 |
| 2 | DCE | 5 | 30 | >99 | 93 |
| 3 | PhCl | 5 | 30 | 58 | 93 |
| 4 | Toluene | 5 | 30 | 52 | 69 |
| 5 | o-Xylene | 5 | 30 | 34 | 85 |
| 6 | THF | 5 | 30 | 0 | — |
| 7 | EtOAc | 5 | 30 | 5 | 84 |
| 8 | MeOH | 5 | 30 | 0 | — |
| 9 | DCE | 4 | 30 | 93 | 91 |
| 10 | DCE | 6 | 30 | >99 | 91 |
| 11 | DCE | 5 | 20 | 96 | 90 |
| 12 | DCE | 5 | 50 | >99 | 90 |
| 13d | DCE | 5 | 30 | 82 | 93 |
| [1] |
(a) Barbachyn, M. R.; Ford, W. Angew. Chem., Int. Ed. 2003, 42, 2010.
doi: 10.1002/anie.200200528 pmid: 15700955 |
|
(b) Mukhtar, T. A.; Wright, G. D. Chem. Rev. 2005, 105, 529.
pmid: 15700955 |
|
|
(c) Wan, Y.-D.; Chen, Z.-X.; Yang, G.-C. Chin. J. Org. Chem. 2005, 25, 1039. (in Chinese)
pmid: 15700955 |
|
|
( 万亚东, 陈祖兴, 杨桂春, 有机化学, 2005, 25, 1039.)
pmid: 15700955 |
|
|
(d) Rensol, A. R.; Luehr, G. W.; Gordeev, M. F. Bioorg. Med. Chem. 2006, 14, 4227.
doi: 10.1016/j.bmc.2006.01.068 pmid: 15700955 |
|
|
(e) Zappia, G.; Menendez, P.; Misiti, D.; Nevola, L.; Botta, B. Mini-Rev. Med. Chem. 2007, 7, 389.
doi: 10.2174/138955707780363783 pmid: 15700955 |
|
|
(f) Das, B.; Rudra, S.; Yadav, A.; Ray, A.; Pandya, M.; Rattan, A.; Mehta, A. Bioorg. Med. Chem. Lett. 2009, 19, 6424.
doi: 10.1016/j.bmcl.2009.09.054 pmid: 15700955 |
|
|
(g) Liang, F.; Chen, L.; Xing, G. Chin. J. Org. Chem. 2009, 29, 1317. (in Chinese)
pmid: 15700955 |
|
|
( 梁芬芬, 陈力, 邢国文, 有机化学, 2009, 29, 1317.)
pmid: 15700955 |
|
|
(h) Shaw, K. J.; Barbachyn, M. R. Ann. N. Y. Acad. Sci. 2011, 1241, 48.
pmid: 15700955 |
|
|
(i) Zhang, H.-Z.; Zhou, C.-H.; Geng, R.-X.; Ji, Q.-G. Chin. J. Org. Chem. 2011, 31, 1963. (in Chinese)
pmid: 15700955 |
|
|
( 张慧珍, 周成合, 耿蓉霞, 吉庆刚, 有机化学, 2011, 31, 1963.)
pmid: 15700955 |
|
| [2] |
Fujimoto, J.; Okamoto, R.; Noguchi, N. J. Med. Chem. 2017, 60, 8963.
doi: 10.1021/acs.jmedchem.7b01210 pmid: 29023121 |
| [3] |
Slassi, A.; Joseph, B.; Ma, F.; Egle, I.; Clayton, I.; Isaac, M.; Swierczek, K. US 20070275966, 2007.
|
| [4] |
(a) Onodera, G.; Watabe, K.; Matsubara, M.; Oda, K.; Kezuka, S.; Takeuchia, R. Adv. Synth. Catal. 2008, 350, 2725.
doi: 10.1002/adsc.200800333 |
|
(b) Li, W.; Wollenburg, M.; Glorius, F. Chem. Sci. 2018, 9, 6260.
doi: 10.1039/C8SC01869C |
|
| [5] |
Han, X.; Liu, R.; Ji, L. J. Chromatogr. B 2016, 1008, 108.
doi: 10.1016/j.jchromb.2015.11.017 |
| [6] |
Lu, C.; Luo, Z.; Huang, L. Tetrahedron: Asymmetry 2011, 22, 722.
|
| [7] |
Wei, S.; Messerer, R.; Tsogoeva, S. B. Chem.-Eur. J. 2011, 17, 14380.
doi: 10.1002/chem.201102931 |
| [8] |
For selected examples: (a) Puschin, N. A.; Mitic, R. V. Leibigs Ann. Chem. 1937, 532, 300.
pmid: 10814394 |
|
(b) Newma, M. S.; Kutner, A. J. Am. Chem. Soc. 1951, 73, 4199.
doi: 10.1021/ja01153a047 pmid: 10814394 |
|
|
(c) Ben, I. D. J. Am. Chem. Soc. 1956, 78, 4962.
doi: 10.1021/ja01600a042 pmid: 10814394 |
|
|
(d) Dyen, M. E.; Swern, D. Chem. Rev. 1967, 67, 197.
pmid: 10814394 |
|
|
(e) Yoshida, T.; Kambe, N.; Murai, S.; Sonoda, N. Tetrahedron Lett. 1986, 27, 3037.
doi: 10.1016/S0040-4039(00)84710-3 pmid: 10814394 |
|
|
(f) Pridgen, L. N.; Prol, J.; Alexander, B. J. Org. Chem. 1989, 54, 3231.
doi: 10.1021/jo00274a058 pmid: 10814394 |
|
|
(g) Ager, D. J.; Prakash, I.; Schaad, D. R. Chem. Rev. 1996, 96, 835.
doi: 10.1021/cr9500038 pmid: 10814394 |
|
|
(h) Kudo, N.; Taniguchi, M.; Furuta, S.; Sato, K.; Endo, T.; Honma, T. J. Agric. Food Chem. 1998, 46, 5305.
doi: 10.1021/jf980281i pmid: 10814394 |
|
|
(i) Cynamon, M. H.; Klemens, S. P.; Sharpe, C. A.; Chase, S. Antimicrob. Agents Chemother. 1999, 43, 1189.
pmid: 10814394 |
|
|
(j) Wang, G.; Hollingsworth, R. I. Tetrahedron: Asymmetry 2000, 11, 4429.
pmid: 10814394 |
|
|
(k) Gabriele, B.; Salerno, G.; Costa, M.; Chiusoli, G. P. Org. Lett. 2000, 2, 625.
pmid: 10814394 |
|
|
(l) Ariza, X.; Pineda, O.; Urpi, F.; Vilarrasa, J. Tetrahedron Lett. 2001, 42, 4995.
doi: 10.1016/S0040-4039(01)00901-7 pmid: 10814394 |
|
|
(m) Liu, J. M.; Peng, X. G.; Liu, J. H.; Zheng, S. Z.; Sun, W.; Xia, C. G. Tetrahedron Lett. 2007, 48, 929.
doi: 10.1016/j.tetlet.2006.12.028 pmid: 10814394 |
|
|
(n) Patil, Y. P.; Tambade, P. J.; Jagtap, S. R.; Bhanage, B. M. J. Mol. Catal. A: Chem. 2008, 289, 14.
doi: 10.1016/j.molcata.2008.03.019 pmid: 10814394 |
|
| [9] |
(a) Iwama, S.; Katsumura, S. Bull. Chem. Soc. Jpn. 1994, 67, 3363.
doi: 10.1246/bcsj.67.3363 pmid: 11667610 |
|
(b) Tingoli, M.; Testaferri, L.; Temparini, A.; Tiecco, M. J. Org. Chem. 1996, 61, 7085.
pmid: 11667610 |
|
|
(c) Ueno, A.; Kayaki, Y.; Ikariya, T. Green Chem. 2013, 15, 425.
doi: 10.1039/C2GC36414J pmid: 11667610 |
|
|
(d) Khan, A.; Xing, J.; Zhao, J.; Kan, Y.; Zhang, W.; Zhang, Y. J. Chem.-Eur. J. 2015, 21, 120.
doi: 10.1002/chem.201405830 pmid: 11667610 |
|
| [10] |
(a) Lebal, H.; Huard, K.; Lectard, S. J. Am. Chem. Soc. 2005, 127, 14198.
doi: 10.1021/ja0552850 |
|
(b) Barman, D. N.; Nicholas, K. M. Eur. J. Org. Chem. 2011, 2011, 908.
doi: 10.1002/ejoc.201001160 |
|
| [11] |
Wan, N. W.; Tian, J. W.; Zhou, X. Y.; Wang, H. H.; Cui, B. D.; Han, W. Y.; Chen, Y. Z. Adv. Synth. Catal. 2019, 361, 4651.
doi: 10.1002/adsc.201900786 |
| [12] |
(a) Noyori, R.; Takaya, H. Acc. Chem. Res. 1990, 23, 345.
doi: 10.1021/ar00178a005 pmid: 24568181 |
|
(b) Tang, W.; Zhang, X. Chem. Rev. 2003, 103, 3029.
doi: 10.1021/cr020049i pmid: 24568181 |
|
|
(c) Johnson, N. B.; Lennon, I. C.; Moran, P. H.; Raasden, J. Acc. Chem. Res. 2007, 40, 1291.
doi: 10.1021/ar700114k pmid: 24568181 |
|
|
(d) Ma, Y.-H.; Zhang, Y.-J.; Zhang, W.-B. Chin. J. Org. Chem. 2007, 27, 289. (in Chinese)
doi: 10.1002/cjoc.200990046 pmid: 24568181 |
|
|
( 马元辉, 张勇健, 张万斌, 有机化学, 2007, 27, 289.)
pmid: 24568181 |
|
|
(e) Li, Y.; Zheng, Y.; Tian, F.; Zhang, Y.; Zhang, W. Chin. J. Org. Chem. 2009, 29, 1487. (in Chinese)
pmid: 24568181 |
|
|
( 李亚玺, 郑玉林, 田丰涛, 张勇健, 张万斌, 有机化学, 2009, 29, 1487.)
pmid: 24568181 |
|
|
(f) Xie, J. H.; Zhu, S. F.; Zhou, Q. L. Chem. Rev. 2011, 111, 1713.
doi: 10.1021/cr100218m pmid: 24568181 |
|
|
(g) Verendel, J. J.; Pamies, O.; Dieguez, M.; Andersson, P. G. Chem. Rev. 2014, 114, 2130.
doi: 10.1021/cr400037u pmid: 24568181 |
|
|
(h) Xie, J.; Zhou, Q. Acta Chim. Sinica 2014, 72, 778. (in Chinese)
pmid: 24568181 |
|
|
( 谢建华, 周其林, 化学学报, 2014, 72, 778.)
doi: 10.6023/A14050364 pmid: 24568181 |
|
|
(i) Wang, Y.; Zhang, Z.; Zhang, W. Chin. J. Org. Chem. 2015, 35, 528. (in Chinese)
doi: 10.6023/cjoc201502017 pmid: 24568181 |
|
|
( 王英杰, 张振锋, 张万斌, 有机化学, 2015, 35, 528.)
doi: 10.6023/cjoc201502017 pmid: 24568181 |
|
|
(j) Yuan, Q.; Zhang, W. Chin. J. Org. Chem. 2016, 36, 274. (in Chinese)
doi: 10.6023/cjoc201509016 pmid: 24568181 |
|
|
( 袁乾家, 张万斌, 有机化学, 2016, 36, 274.)
doi: 10.6023/cjoc201509016 pmid: 24568181 |
|
|
(k) Wang, Z.; Zhang, Z.; Liu, Y.; Zhang, W. Chin. J. Org. Chem. 2016, 36, 447. (in Chinese)
doi: 10.6023/cjoc201512009 pmid: 24568181 |
|
|
( 王志惠, 张振锋, 刘燕刚, 张万斌, 有机化学, 2016, 36, 447.)
doi: 10.6023/cjoc201512009 pmid: 24568181 |
|
|
(l) Zhang, Z.; Butt, N. A.; Zhang, W. Chem. Rev. 2016, 116, 14769.
doi: 10.1021/acs.chemrev.6b00564 pmid: 24568181 |
|
|
(m) Wiesenfeldt, M. P.; Nairoukh, Z.; Dalton, T.; Glorius, F. Angew. Chem., Int. Ed. 2019, 58, 10460.
doi: 10.1002/anie.201814471 pmid: 24568181 |
|
|
(n) Gu, X.; Li, X.; Xie, J.; Zhou, Q. Acta Chim. Sinica 2019, 77, 598. (in Chinese)
pmid: 24568181 |
|
|
( 顾雪松, 李校根, 谢建华, 周其林, 化学学报, 2019, 77, 598.)
doi: 10.6023/A19050166 pmid: 24568181 |
|
|
(o) Dou, X.; Liu, D.; Zhang, W. Chin. J. Pharm. 2020, 51, 145. (in Chinese)
pmid: 24568181 |
|
|
( 窦骁勇, 刘德龙, 张万斌, 中国医药工业杂志, 2020, 51, 145.)
pmid: 24568181 |
|
|
(p) Chen, J.; Zhang, W. Chin. J. Org. Chem. 2020, 40, 4372. (in Chinese)
doi: 10.6023/cjoc202000086 pmid: 24568181 |
|
|
( 陈建中, 张万斌, 有机化学, 2020, 40, 4372.)
doi: 10.6023/cjoc202000086 pmid: 24568181 |
|
|
(q) Zheng, Y.; Zhang, X.; Zhang, R.; Ma, B. Green Synth. Catal. 2021, 2, 393.
pmid: 24568181 |
|
| [13] |
Wang, Q.; Tan, X.; Zhu, Z.; Dong, X. Q.; Zhang, X. Tetrahedron Lett. 2016, 57, 658.
doi: 10.1016/j.tetlet.2015.12.105 |
| [14] |
Li, W.; Wollenburg, M.; Glorius, F. Chem. Sci. 2018, 9, 6260.
doi: 10.1039/C8SC01869C |
| [15] |
Liu, Y.; Yi, Z.; Yang, X.; Wang, H.; Yin, C.; Wang, M.; Dong, X. Q.; Zhang, X. ACS Catal. 2020, 10, 11153.
doi: 10.1021/acscatal.0c02569 |
| [16] |
(a) Tian, F.; Yao, D.; Zhang, Y. J.; Zhang, W. Tetrahedron 2009, 65, 9609.
doi: 10.1016/j.tet.2009.09.053 pmid: 34213913 |
|
(b) Tian, F.; Yao, D.; Liu, Y.; Xie, F.; Zhang, W. Adv. Synth. Catal. 2010, 352, 1841.
doi: 10.1002/adsc.201000185 pmid: 34213913 |
|
|
(c) Liu, Y.; Yao, D.; Li, K.; Tian, F.; Xie, F.; Zhang, W. Tetrahedron 2011, 67, 8445.
doi: 10.1016/j.tet.2011.09.023 pmid: 34213913 |
|
|
(d) Liu, Y.; Zhang, W. Angew. Chem., Int. Ed. 2013, 52, 2203.
doi: 10.1002/anie.201209126 pmid: 34213913 |
|
|
(e) Liu, Y.; Gridnev, I. D.; Zhang, W. Angew. Chem., Int. Ed. 2014, 53, 1901.
doi: 10.1002/anie.201309677 pmid: 34213913 |
|
|
(f) Xia, J.; Yang, G.; Zhuge, R.; Liu, Y.; Zhang, W. Chem. Eur. J. 2016, 22, 18354.
doi: 10.1002/chem.201604298 pmid: 34213913 |
|
|
(g) Quan, M.; Tang, L.; Shen, J.; Yang, G.; Zhang, W. Chem. Commun. 2017, 53, 609.
doi: 10.1039/C6CC08759K pmid: 34213913 |
|
|
(h) Xia, J.; Nie, Y.; Yang, G.; Liu, Y.; Zhang, W. Org. Lett. 2017, 19, 4884.
doi: 10.1021/acs.orglett.7b02341 pmid: 34213913 |
|
|
(i) Meng, K.; Xia, J.; Wang, Y.; Zhang, X.; Yang, G.; Zhang, W. Org. Chem. Front. 2017, 4, 1601.
doi: 10.1039/C7QO00248C pmid: 34213913 |
|
|
(j) Xia, J.; Nie, Y.; Yang, G.; Liu, Y.; Gridnev, I. D.; Zhang, W. Chin. J. Chem. 2018, 36, 612.
doi: 10.1002/cjoc.201800088 pmid: 34213913 |
|
|
(k) Wang, Y.; Xia, J.; Yang, G.; Zhang, W. Tetrahedron 2018, 74, 477.
doi: 10.1016/j.tet.2017.12.015 pmid: 34213913 |
|
|
(l) Yan, J.; Nie, Y.; Gao, F.; Yuan, Q.; Xie, F.; Zhang, W. Tetrahedron 2021, 84, 132003.
doi: 10.1016/j.tet.2021.132003 pmid: 34213913 |
|
|
(m) Nie, Y.; Li, J.; Yan, J.; Yuan, Q.; Zhang, W. Org. Lett. 2021, 23, 5373.
doi: 10.1021/acs.orglett.1c01701 pmid: 34213913 |
|
| [17] |
Tobias, A.; Goran, H. Org. Lett. 2009, 11, 503.
doi: 10.1021/ol802243d pmid: 19123840 |
| [1] | Qian Wang, Yuqi Liu, Zongquan Wu. Synthesis and Structure Control of Chiral Helical Polymers [J]. Chinese Journal of Organic Chemistry, 2023, 43(12): 4141-4146. |
| [2] | Jiayi Zhao, Yicong Ge, Chuan He. Construction of Silicon-Stereogenic Center via Catalytic Asymmetric Si—H/X—H Dehydrogenative Coupling [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3352-3366. |
| [3] | Jieming Zhang, Hang Ni, Qi Wu, Junfeng Yang, Junliang Zhang. Development of P, S and Si-Stereogenic Compounds Synthesis via Palladium Catalysis [J]. Chinese Journal of Organic Chemistry, 2022, 42(10): 3118-3128. |
| [4] | Sha Zhou, Mukuo Wang, Weibin Xie, Shaa Zhou, Lixia Xiong, Yu Zhao, Zhengming Li. Synthesis and Insecticidal Activities of Novel Optically Active Dicarboxamides Containing N-Trifluoroacetyl Sulfulimiyl Substituents [J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3532-3538. |
| [5] | Min Zhou, Jing Li, Jie Cheng, Congwu Ge, Tanyu Cheng, Xike Gao. Synthesis and Field-Effect Characteristics of the Chiral Naphthalene Diimide Derivatives [J]. Chinese Journal of Organic Chemistry, 2021, 41(11): 4400-4408. |
| [6] | Zhang Yongna, Duan Hui-Xin, Wang You-Qing. Research Progress in Asymmetric Reactions of Imines Using Chiral Primary Amines as Organocatalysts [J]. Chinese Journal of Organic Chemistry, 2020, 40(6): 1514-1528. |
| [7] | Zhang Shuo, Liao Gang, Shi Bingfeng. Enantioselective Synthesis of Atropisomers Featuring Pentatomic Heteroaromatics [J]. Chin. J. Org. Chem., 2019, 39(6): 1522-1528. |
| [8] | Yang Yun, Liu Huihui, Liu Xiaobing, Liu Tiantian, Zhu Yuqin, Zhang Anan, Wang Tao, Hua Yuanzhao, Wang Mincan, Mao Guoliang, Liu Lantao. Asymmetric Synthesis of Axial Chiral Vinylarenes Fearturing Oxindole Moiety via Tandem Carbopalladation/C-H Olefination [J]. Chin. J. Org. Chem., 2019, 39(6): 1655-1664. |
| [9] | Yang Sen, Ding Chengrong, Liu Xinghai, Weng Jianquan, Yuan Jing, Tan Chengxia. Synthesis and Herbicidal Activity of Chiral Aryloxyphenoxypropionic Amides Compounds [J]. Chinese Journal of Organic Chemistry, 2019, 39(12): 3588-3593. |
| [10] | Cai Zhengjun, Gao Jianbao, Li Bai, Zhong Yuan, Feng Xing, Xue Jijun, Jiang Xianxing. Application of [4+2]Cycloaddition Reaction of Tetrazine with Cyclooctyne in the Construction of Pyridazine Structure with Axial Chirality [J]. Chin. J. Org. Chem., 2018, 38(5): 1138-1146. |
| [11] | Sun Yunkai, Zhang Jin, Liu Huijun, Wang Xiaofeng. Synthesis and Polymerization of Optically Active Epoxides with Diazafluorenyl Substituent [J]. Chin. J. Org. Chem., 2018, 38(1): 253-258. |
| [12] | Huang Jiapian, Gu Qing, You Shuli. Synthesis of Planar Chiral Ferrocenes via Transition-Metal-Catalyzed Direct C-H Bond Functionalization [J]. Chin. J. Org. Chem., 2018, 38(1): 51-61. |
| [13] | Zhu Xinju, Niu Junlong, Zhao Xuemei, Hao Xinqi, Song Maoping. Synthesis of Chiral Bis(imidazoline) Ligands with Biphenyl Backbone and Their Application in the Asymmetric Cyclopropanation Reaction [J]. Chin. J. Org. Chem., 2018, 38(1): 118-123. |
| [14] | Yan Yugang, Chen Xueying, Yang Xinying, Xu Wenfang, Zhang Yingjie. Sulfoxide Analogs of TAK-875 as G Protein Coupled Receptor 40 Agonists: Synthesis, Determination of Absolute Configuration and Biological Activity [J]. Chin. J. Org. Chem., 2017, 37(4): 858-865. |
| [15] | Fu Liyan, Ji Baoming, Du Chenxia. Synthesis of a New Class of Chiral Maleimide Derivatives with C2-Symmetry [J]. Chin. J. Org. Chem., 2017, 37(10): 2685-2689. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||