Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (11): 4400-4408.DOI: 10.6023/cjoc202105023 Previous Articles Next Articles
ARTICLES
周敏a,b, 李晶b, 程杰b, 葛从伍b,*( ), 程探宇a,*(
), 程探宇a,*( ), 高希珂b,*(
), 高希珂b,*( )
)
收稿日期:2021-05-12
修回日期:2021-07-15
发布日期:2021-08-17
通讯作者:
葛从伍, 程探宇, 高希珂
基金资助:
Min Zhoua,b, Jing Lib, Jie Chengb, Congwu Geb( ), Tanyu Chenga(
), Tanyu Chenga( ), Xike Gaob(
), Xike Gaob( )
)
Received:2021-05-12
Revised:2021-07-15
Published:2021-08-17
Contact:
Congwu Ge, Tanyu Cheng, Xike Gao
Supported by:Share
Min Zhou, Jing Li, Jie Cheng, Congwu Ge, Tanyu Cheng, Xike Gao. Synthesis and Field-Effect Characteristics of the Chiral Naphthalene Diimide Derivatives[J]. Chinese Journal of Organic Chemistry, 2021, 41(11): 4400-4408.
| Compd. | λmax/nm | Ega/eV | Tdb/℃ | Tptc/℃ | |
|---|---|---|---|---|---|
| Solid | Film | Heating/Cooling | |||
| I-R | 630 | 639 | 1.78 | 416 | 277/283 |
| I-S | 630 | 639 | 1.78 | 416 | 277/283 |
| I-R,S | 630 | 644 | 1.78 | 418 | 277/287 |
| I-rac | 630 | 644 | 1.78 | 424 | 285/292 |
| Compd. | λmax/nm | Ega/eV | Tdb/℃ | Tptc/℃ | |
|---|---|---|---|---|---|
| Solid | Film | Heating/Cooling | |||
| I-R | 630 | 639 | 1.78 | 416 | 277/283 |
| I-S | 630 | 639 | 1.78 | 416 | 277/283 |
| I-R,S | 630 | 644 | 1.78 | 418 | 277/287 |
| I-rac | 630 | 644 | 1.78 | 424 | 285/292 |
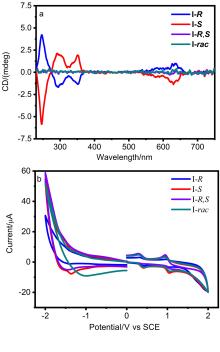

| Compd. | Annealing temperature/℃ | µh,max/ (cm2•V–1•s–1) | VT/V | IOn/IOff |
|---|---|---|---|---|
| I-R | As-spun | 3.2×10–5 | 6.1 | 102~103 |
| 100 | 4.0×10–2 | –62.9 | 104~105 | |
| 180 | 4.6×10–3 | –40.4 | 103~104 | |
| I-S | As-spun | 3.8×10–5 | 1.8 | 102~103 |
| 100 | 3.6×10–2 | –62.4 | 104~105 | |
| 180 | 4.6×10–3 | –42.6 | 103~104 | |
| I-R,S | As-spun | 4.9×10–5 | 2.9 | 102~103 |
| 100 | 7.4×10–2 | –50.6 | 104~105 | |
| 180 | 1.4×10–2 | –40.0 | 104~105 | |
| I-rac | As-spun | 1.7×10–5 | 3.0 | 102~103 |
| 100 | 7.5×10–2 | –62.5 | 104~105 | |
| 180 | 1.5×10–2 | –37.0 | 104~105 |
| Compd. | Annealing temperature/℃ | µh,max/ (cm2•V–1•s–1) | VT/V | IOn/IOff |
|---|---|---|---|---|
| I-R | As-spun | 3.2×10–5 | 6.1 | 102~103 |
| 100 | 4.0×10–2 | –62.9 | 104~105 | |
| 180 | 4.6×10–3 | –40.4 | 103~104 | |
| I-S | As-spun | 3.8×10–5 | 1.8 | 102~103 |
| 100 | 3.6×10–2 | –62.4 | 104~105 | |
| 180 | 4.6×10–3 | –42.6 | 103~104 | |
| I-R,S | As-spun | 4.9×10–5 | 2.9 | 102~103 |
| 100 | 7.4×10–2 | –50.6 | 104~105 | |
| 180 | 1.4×10–2 | –40.0 | 104~105 | |
| I-rac | As-spun | 1.7×10–5 | 3.0 | 102~103 |
| 100 | 7.5×10–2 | –62.5 | 104~105 | |
| 180 | 1.5×10–2 | –37.0 | 104~105 |


| [1] |
Kuang, H.; Xu, C.; Tang, Z. Adv. Mater. 2020, 32, 2005110.
doi: 10.1002/adma.v32.41 |
| [2] |
Liu, M. Acta Phys. Chim. Sin. 2020, 36, 2004031. (in Chinese)
|
|
(刘鸣华, 物理化学学报, 2020, 36, 2004031.)
|
|
| [3] |
Liu, J.; Yin, F.; Hu, J.; Ju, Y. Chin. J. Org. Chem. 2021, 41, 1031. (in Chinese)
doi: 10.6023/cjoc202008011 |
|
(刘金果, 殷凤, 胡君, 巨勇, 有机化学, 2021, 41, 1031.)
doi: 10.6023/cjoc202008011 |
|
| [4] |
Pasteur, L. Ann. Chim. Phys. 1848, 24, 442.
|
| [5] |
Reinitzer, F. Monatsh. Chem. 1888, 9, 421.
doi: 10.1007/BF01516710 |
| [6] |
Geng, Y.; Trajkovska, A.; Culligan, S. W.; Ou, J. J.; Chen, H. M. P.; Katsis, D.; Chen, S. H. J. Am. Chem. Soc. 2003, 125, 14032.
doi: 10.1021/ja037733e |
| [7] |
Yang, Y.; da Costa, R. C.; Smilgies, D. M.; Campbell, A. J.; Fuchter, M. J. Adv. Mater. 2013, 25, 2624.
doi: 10.1002/adma.v25.18 |
| [8] |
Liu, J.; Su, H.; Meng, L.; Zhao, Y.; Deng, C.; Ng, J. C. Y.; Lu, P.; Faisal, M.; Lam, J. W. Y.; Huang, X.; Wu, H.; Wong, K. S.; Tang, B. Chem. Sci. 2012, 3, 2737.
doi: 10.1039/c2sc20382k |
| [9] |
Hatakeyama, T.; Hashimoto, S.; Oba, T.; Nakamura, M. J. Am. Chem. Soc. 2012, 134, 19600.
doi: 10.1021/ja310372f pmid: 23167918 |
| [10] |
Liu, J.; Zhang, Y.; Phan, H.; Sharenko, A.; Moonsin, P.; Walker, B.; Promarak, V.; Nguyen, T. Q. Adv. Mater. 2013, 25, 3645.
doi: 10.1002/adma.v25.27 |
| [11] |
Ying, Y.; Rice, B.; Shi, X.; Brandt, J. R.; Correa da Costa, R.; Hedley, G. J.; Smilgies, D. M.; Frost, J. M.; Samuel, I. D. W.; Otero-de-la-Roza, A.; Johnson, E. R.; Jelfs, K. E.; Nelson, J.; Campbell, A. J.; Fuchter, M. J. ACS Nano 2017, 11, 8329.
doi: 10.1021/acsnano.7b03540 pmid: 28696680 |
| [12] |
Chen, M.; Jiao, X. C.; Li, J.; Wu, W.; Xin, H.; McNeill, C. R.; Gao, X. Langmuir 2019, 35, 6188.
doi: 10.1021/acs.langmuir.9b00463 |
| [13] |
Sakai, N.; Mareda, J.; Vauthey, E.; Matile. Chem. Commun. 2010, 46, 4225.
doi: 10.1039/c0cc00078g |
| [14] |
Bhosale, S. V.; Bhosale, S. V.; Bhargava, S. K. Org. Biomol. Chem. 2012, 10, 6455.
doi: 10.1039/c2ob25798j |
| [15] |
Suraru, S. L.; Würthner, F. Angew. Chem., nt. Ed. 2014, 53, 7428.
|
| [16] |
Guo, D.; Li, L.; Zhu, X.; Heeney, M.; Li, J.; Dong, L.; Yu, Q.; Gan, Z.; Gu, X.; Tan, L. Sci. China Chem. 2020, 63, 1198.
doi: 10.1007/s11426-020-9776-8 |
| [17] |
Zhang, F.; Hu, Y.; Schuettfort, T.; Di, C.; Gao, X.; McNeill, C. R.; Thomsen, L.; Mannsfeld, S. C. B.; Yuan, W.; Sirringhaus, H.; Zhu, D. J. Am. Chem. Soc. 2013, 135, 2338.
doi: 10.1021/ja311469y |
| [18] |
Zhao, Z.; Zhang, F. J.; Hu, Y.; Wang, Z.; Leng, B.; Gao, X.; Di, C.; Zhu, D. ACS Macro Lett. 2014, 3, 1174.
doi: 10.1021/mz500603f |
| [19] |
Wu, W.; Zhao, Z.; Li, J.; Chen, M.; Gao, X. Asian J. Org. Chem. 2018, 7, 2279.
doi: 10.1002/ajoc.201800443 |
| [20] |
Han, W.; Wang, Z.; Hu, Y.; Yang, X.; Ge, C.; Gao, X. Sci. China Chem. 2020, 63, 1182.
doi: 10.1007/s11426-020-9792-3 |
| [21] |
Sasikumar, M.; Suseela, Y. V.; Govindaraju, T. Asian J. Org. Chem. 2013, 2, 779.
doi: 10.1002/ajoc.v2.9 |
| [22] |
Hu, Y.; Wang, Z.; Yang, X.; Zhao, Z.; Han, W.; Yuan, W.; Li, H.; Gao, X.; Zhu, D. Tetrahedron Lett. 2013, 54, 2271.
doi: 10.1016/j.tetlet.2013.02.075 |
| [23] |
Jelley, E. E. Nature 1936, 138, 1009.
doi: 10.1038/1381009a0 |
| [24] |
Gao, X.; Qiu, W.; Yang, X.; Liu, Y.; Wang, Y.; Zhang, H.; Qi, T.; Liu, Y.; Lu, K.; Du, C.; Shuai, Z.; Yu, G.; Zhu, D. Org. Lett. 2007, 9, 3917
doi: 10.1021/ol701539z |
| [25] |
Röger, C.; Würthner, F. J. Org. Chem. 2007, 72, 8070.
doi: 10.1021/jo7015357 |
| [26] |
Gao, X.; Qiu, W.; Yang, X.; Liu, Y.; Wang, Y.; Zhang, H.; Qi, H.; Liu, Y.; Lu, K.; Du, C.; Shuai, Z.; Yu, G.; Zhu, D. Org. Lett. 2007, 9, 3917
doi: 10.1021/ol701539z |
| [1] | Qian Wang, Yuqi Liu, Zongquan Wu. Synthesis and Structure Control of Chiral Helical Polymers [J]. Chinese Journal of Organic Chemistry, 2023, 43(12): 4141-4146. |
| [2] | Jiayi Zhao, Yicong Ge, Chuan He. Construction of Silicon-Stereogenic Center via Catalytic Asymmetric Si—H/X—H Dehydrogenative Coupling [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3352-3366. |
| [3] | Tongli Zhang, Jun Yan, Jingli He, Xuezhen Kou, Jiefeng Shen, Delong Liu, Wanbin Zhang. Synthesis of Chiral 5-Aryl-2-oxazolidinones via an Ir-BiphPHOX Catalyzed Enantioselective Hydrogenation [J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1747-1758. |
| [4] | Jieming Zhang, Hang Ni, Qi Wu, Junfeng Yang, Junliang Zhang. Development of P, S and Si-Stereogenic Compounds Synthesis via Palladium Catalysis [J]. Chinese Journal of Organic Chemistry, 2022, 42(10): 3118-3128. |
| [5] | Min Li, Aifeng Lv. Recent Progress in Ambipolar Organic Field-Effect Transistors Based on Organic Semiconductor Bilayer [J]. Chinese Journal of Organic Chemistry, 2022, 42(1): 54-66. |
| [6] | Sha Zhou, Mukuo Wang, Weibin Xie, Shaa Zhou, Lixia Xiong, Yu Zhao, Zhengming Li. Synthesis and Insecticidal Activities of Novel Optically Active Dicarboxamides Containing N-Trifluoroacetyl Sulfulimiyl Substituents [J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3532-3538. |
| [7] | Zhang Yongna, Duan Hui-Xin, Wang You-Qing. Research Progress in Asymmetric Reactions of Imines Using Chiral Primary Amines as Organocatalysts [J]. Chinese Journal of Organic Chemistry, 2020, 40(6): 1514-1528. |
| [8] | Yang Yun, Liu Huihui, Liu Xiaobing, Liu Tiantian, Zhu Yuqin, Zhang Anan, Wang Tao, Hua Yuanzhao, Wang Mincan, Mao Guoliang, Liu Lantao. Asymmetric Synthesis of Axial Chiral Vinylarenes Fearturing Oxindole Moiety via Tandem Carbopalladation/C-H Olefination [J]. Chin. J. Org. Chem., 2019, 39(6): 1655-1664. |
| [9] | Zhang Shuo, Liao Gang, Shi Bingfeng. Enantioselective Synthesis of Atropisomers Featuring Pentatomic Heteroaromatics [J]. Chin. J. Org. Chem., 2019, 39(6): 1522-1528. |
| [10] | Yang Sen, Ding Chengrong, Liu Xinghai, Weng Jianquan, Yuan Jing, Tan Chengxia. Synthesis and Herbicidal Activity of Chiral Aryloxyphenoxypropionic Amides Compounds [J]. Chinese Journal of Organic Chemistry, 2019, 39(12): 3588-3593. |
| [11] | Liang Long, Liu Li-Na, Chen Xue-Qiang, Xiang Xuan, Ling Jun, Lu Zheng-Quan, Li Jing-Jing, Li Wei-Shi. Benzodithiophene/Benzothiadiazole-Based ADA-Type Optoelectronic Molecules: Influence of Fluorine Substitution [J]. Chin. J. Org. Chem., 2019, 39(1): 157-169. |
| [12] | Cai Zhengjun, Gao Jianbao, Li Bai, Zhong Yuan, Feng Xing, Xue Jijun, Jiang Xianxing. Application of [4+2]Cycloaddition Reaction of Tetrazine with Cyclooctyne in the Construction of Pyridazine Structure with Axial Chirality [J]. Chin. J. Org. Chem., 2018, 38(5): 1138-1146. |
| [13] | Huang Jiapian, Gu Qing, You Shuli. Synthesis of Planar Chiral Ferrocenes via Transition-Metal-Catalyzed Direct C-H Bond Functionalization [J]. Chin. J. Org. Chem., 2018, 38(1): 51-61. |
| [14] | Zhu Xinju, Niu Junlong, Zhao Xuemei, Hao Xinqi, Song Maoping. Synthesis of Chiral Bis(imidazoline) Ligands with Biphenyl Backbone and Their Application in the Asymmetric Cyclopropanation Reaction [J]. Chin. J. Org. Chem., 2018, 38(1): 118-123. |
| [15] | Sun Yunkai, Zhang Jin, Liu Huijun, Wang Xiaofeng. Synthesis and Polymerization of Optically Active Epoxides with Diazafluorenyl Substituent [J]. Chin. J. Org. Chem., 2018, 38(1): 253-258. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||