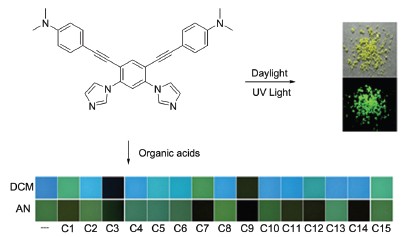
| [1] Pugh, D.; Danopoulos, A. A. Coord. Chem. Rev. 2007, 251, 610. [2] Poyatos, M.; Mata, J. A.; Peris, E. Chem. Rev. 2009, 109, 3677. [3] Cho, J.; Hollis, T. K.; Helgert, T. R.; Valente, E. J. Chem. Commun. 2008, 5001. [4] Bauer, E. B.; Andavan, G. T. S.; Hollis, T. K.; Rubio, R. J.; Cho, J.; Kuchenbeiser, G. R.; Helgert, T. R.; Letko, C. S.; Tham, F. S. Org. Lett. 2008, 10, 1175. [5] Senthil, A. G. T.; Bauer, E. B.; Letko, C. S.; Hollis, T. K.; Tham, F. S. J. Organomet. Chem. 2005, 690, 5938. [6] Gu, Z.-G.; Wang, B.-X.; Pang, C.-Y.; Zhou, W.; Li, Z.-J. Acta Chim. Sinica 2012, 70, 2501. (顾志国, 王宝祥, 庞春燕, 周文, 李在均, 化学学报, 2012, 70, 2501.)[7] Zhang, X.-F.; Wright, A. M.; DeYonker, N. J.; Hollis, T. K.; Hammer, N. I.; Webster, C. E.; Valente, E. J. Organometallics 2012, 31, 1664. [8] Chung, L.-H.; Chan, S.-C.; Lee, W.-C.; Wong, C.-Y. Inorg. Chem. 2012, 51, 8693. [9] Zhou, H.-J.; Wang, Z.; Gao, C.; You, J.-S.; Gao, G. Chem. Commun. 2013, 49, 1832. [10] Schlechte, L.; Bon, V.; Grünker, R.; Klein, N.; Senkovska, I.; Kaskel, S. Polyhedron 2012, 44, 179. [11] Su, Z.; Fan, J.; Okamura, T.; Sun, W.-Y.; Ueyama, N. Cryst. Growth Des. 2010, 10, 3515. [12] Fan, J.; Gan, L.; Gan, K. H.; Sun, W.-Y.; Yu, K.-B.; Tang, W.-X. Chem. Eur. J. 2003, 9, 3965. [13] Zhang, S.-Y.; Yang, S.-Y.; Lan, J.-B.; Yang, S.-J.; You, J.-S. Chem. Commun. 2008, 6170. [14] Wang, H.-Y.; Feng, J.-C.; Jiang, H.-J.; Huang, W.; Wei, W. Prog. Chem. 2007, 19, 276. (王洪宇, 冯嘉春, 姜鸿基, 黄维, 韦玮, 化学进展, 2007, 19, 276.)[15] Wilson, J. N.; Bunz, U. H. F. J. Am. Chem. Soc. 2005, 127, 4124. [16] Spitler, E. L.; Shirtcliff, L. D.; Haley, M. M. J. Org. Chem. 2007, 72, 86. [17] Davey, E. A.; Zucchero, A. J.; Trapp, O.; Bunz, U. H. F. J. Am. Chem. Soc. 2011, 133, 7716. [18] Lim, J.; Nam, D.; Miljanic, O. S. Chem. Sci. 2012, 3, 559. [19] Zhang, L.-Q.; Gu, W.-J.; Chen, C.-X.; Wang, B.-X. Acta Chim. Sinica 2010, 68, 1981. (张林群, 顾玮瑾, 陈长信, 王炳祥, 化学学报, 2010, 68, 1981.)[20] Shimizu, M.; Hiyama, T. Chem. Asian J. 2010, 5, 1516. [21] Luo, J.-D.; Xie, Z.-L.; Lam, J. W. Y.; Lam, C. L.; Chen, H.-Y.; Qiu, C.-F.; Kwok, H. S.; Zhan, X.-W.; Liu, Y.-Q.; Zhu, D.-B.; Tang, B.-Z. Chem. Commun. 2001, 1740. [22] Hong, Y.-N.; Lamab, J. W. Y.; Tang, B.-Z. Chem. Soc. Rev. 2011, 40, 5361. [23] Zhang, S.; Qin, A.-J.; Sun, J.-Z.; Tang, B.-Z. Prog. Chem. 2011, 23, 623. (张双, 秦安军, 孙景志, 唐本忠, 化学进展, 2011, 23, 623.)[24] Available at: http://cbc.arizona.edu/njardarson/group/top-pharma- ceuticals-poster. [25] Li, D.; Gao, B.-J.; Xu, W.-M. Acta Chim. Sinica 2011, 69, 3019. (李丁, 高保娇, 许文梅, 化学学报, 2011, 69, 3019.)[26] Schwaebel, T.; Trapp, O.; Bunz, U. H. F. Chem. Sci. 2013, 4, 273. [27] Yu, X.-D.; Liu, Q.; Xu, X.-F.; Lan, H.-C.; Cao, X.-H.; Chen, L.-M.; Liu, B.; Yi, T. Acta Chim. Sinica 2012, 70, 2016. (余旭东, 刘倩, 许秀芳, 兰海闯, 曹新华, 陈黎明, 刘斌, 易涛, 化学学报, 2012, 70, 2016.)[28] Hart, H.; Harada, K.; Frank Du, C.-J. J. Org. Chem. 1985, 50, 3104. [29] Jian, H.-H.; Tour, J. M. J. Org. Chem. 2003, 68, 5091. |