化学学报 ›› 2019, Vol. 77 ›› Issue (9): 911-915.DOI: 10.6023/A19050181 上一篇 下一篇
所属专题: 有机自由基化学
研究通讯
投稿日期:2019-05-15
发布日期:2019-08-14
通讯作者:
刘强
E-mail:liuqiang@lzu.edu.cn
基金资助:
Dai, Jianling, Lei, Wenlong, Liu, Qiang*( )
)
Received:2019-05-15
Published:2019-08-14
Contact:
Liu, Qiang
E-mail:liuqiang@lzu.edu.cn
Supported by:文章分享
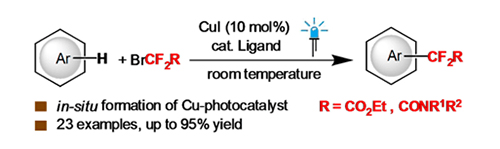
以CuI为铜源, 通过原位形成光催化剂的途径, 实现了室温下可见光驱使铜催化溴二氟乙酸乙酯、溴二氟酰胺等对芳烃及杂芳烃的二氟烷基化反应. 该反应条件温和、原料廉价易得、底物适用范围广、产率较高, 为合成二氟烷基(杂)芳烃化合物提供了一种方法. 机理研究表明, 该反应可能经历了单电子转移的自由基反应历程.
戴建玲, 雷文龙, 刘强. 可见光驱使铜盐催化芳香烃二氟烷基化反应[J]. 化学学报, 2019, 77(9): 911-915.
Dai, Jianling, Lei, Wenlong, Liu, Qiang. Visible-Light-Driven Difluoroalkylation of Aromatics Catalyzed by Copper[J]. Acta Chimica Sinica, 2019, 77(9): 911-915.
| Entry | Changes from the "standard conditions" | Yielda |
|---|---|---|
| 1 | No changes | 87 |
| 2 | Without CuI, K3PO4, or L5 | 0 |
| 3 | Without L1 | 40 |
| 4 | Cu(OAc)2 instead of CuI | 0 |
| 5 | Cu(MeCN)4PF6 instead of CuI | 85 |
| 6 | KOAc instead of K3PO4 | 77 |
| 7 | K2CO3 instead of K3PO4 | 86 |
| 8 | CH3CN instead of DCM | 61 |
| 9 | CH3OH instead of DCM | 21 |
| 10 | DMF instead of DCM | 40 |
| 11 | 2.0 equiv. 1a | 80 |
| 12 | L2 instead of L1 | 95 |
| 13 | L3 instead of L1 | 0 |
| 14 | L4 instead of L1 | 0 |
| 15 | L6 instead of L5 | 0 |
| 16 | [Cu(dcp)(xantphos)]I (10 mol%) | 92 |
| 17 | CuI (5 mol%), L2 (5 mol%) and L5 (5 mol%) | 88 |
| 18 | 1.0 equiv. K3PO4 | 30 |
| 19 | under air (open flask) | 0 |
| 20 | dark | 0 |
| Entry | Changes from the "standard conditions" | Yielda |
|---|---|---|
| 1 | No changes | 87 |
| 2 | Without CuI, K3PO4, or L5 | 0 |
| 3 | Without L1 | 40 |
| 4 | Cu(OAc)2 instead of CuI | 0 |
| 5 | Cu(MeCN)4PF6 instead of CuI | 85 |
| 6 | KOAc instead of K3PO4 | 77 |
| 7 | K2CO3 instead of K3PO4 | 86 |
| 8 | CH3CN instead of DCM | 61 |
| 9 | CH3OH instead of DCM | 21 |
| 10 | DMF instead of DCM | 40 |
| 11 | 2.0 equiv. 1a | 80 |
| 12 | L2 instead of L1 | 95 |
| 13 | L3 instead of L1 | 0 |
| 14 | L4 instead of L1 | 0 |
| 15 | L6 instead of L5 | 0 |
| 16 | [Cu(dcp)(xantphos)]I (10 mol%) | 92 |
| 17 | CuI (5 mol%), L2 (5 mol%) and L5 (5 mol%) | 88 |
| 18 | 1.0 equiv. K3PO4 | 30 |
| 19 | under air (open flask) | 0 |
| 20 | dark | 0 |
| [1] |
(a) Müller, K.; Faeh, C.; Diederich, F . Science. 2007, 317, 1881.
doi: 10.1126/science.1131943 |
|
(b) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V . Chem. Soc. Rev. 2008, 37, 320.
doi: 10.1126/science.1131943 |
|
| [2] |
(a) Jeschke, P . ChemBioChem. 2004, 5, 570.
doi: 10.1002/cbic.v5:5 |
|
(b) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V . Chem. Soc. Rev. 2008, 37, 320.
doi: 10.1002/cbic.v5:5 |
|
|
(c) Wang, J.; Sanchez-Rosello, M.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H . Chem. Rev. 2014, 114, 2432.
doi: 10.1002/cbic.v5:5 |
|
| [3] |
(a) Bégué, J. P.; Bonnet-Delpon, D . J. Fluorine Chem. 2006, 127, 992.
doi: 10.1016/j.jfluchem.2006.05.006 |
|
(b) Isanbor, C . J. Fluorine Chem. 2006, 127, 303.
doi: 10.1016/j.jfluchem.2006.05.006 |
|
|
(c) Kirk, K. L . J. Fluorine Chem. 2006, 127, 1013.
doi: 10.1016/j.jfluchem.2006.05.006 |
|
| [4] |
Wong, D. T.; Bymaster, F. P.; Engleman, E. A . Life Sci. 1995, 57, 411.
doi: 10.1016/0024-3205(95)00209-O |
| [5] | Roth, B. D . In Progress in Medicinal Chemistry, Vol. 40, Eds.: King, F. D.; Oxford, A. W., Elsevier, Amsterdam, 2002, pp. 1~ 22. |
| [6] |
Drlica, K.; Malik, M . Curr. Top. Med. Chem. 2003, 3, 249.
doi: 10.2174/1568026033452537 |
| [7] |
(a) Purser, S.; Moore, P.-R.; Swallow, S.; Gouverneur, V . Chem. Soc. Rev. 2008, 37, 320.
doi: 10.1039/B610213C |
|
(b) Nenajdenko, V. G.; Shastin, A. V . Chem. Rev. 2015, 115, 973.
doi: 10.1039/B610213C |
|
|
(c) Ni, C.-F.; Hu, J.-B . Chem. Rev. 2015, 115, 765.
doi: 10.1039/B610213C |
|
|
(d) Liang, T.; Ritter, T . Angew. Chem., Int. Ed. 2013, 52, 8214.
doi: 10.1039/B610213C |
|
|
(e) Zhou, B.-Y.; Cheng, J.-P . Org. Lett. 2016, 18, 6128.
doi: 10.1039/B610213C |
|
|
(f) Yu, W.; Qing, F.-L . Org. Lett. 2016, 18, 5130.
doi: 10.1039/B610213C |
|
|
(g) Guo, W.-H.; Zhang, X . ACS Catal. 2017, 7, 896.
doi: 10.1039/B610213C |
|
|
(h) Fu, X.-P.; Xiao, Y.-L.; Zhang, X . Chin. J. Chem. 2018, 36, 143.
doi: 10.1039/B610213C |
|
|
(i) He, X.; Gao, X.; Zhang, X . Chin. J. Chem. 2018, 36, 1059.
doi: 10.1039/B610213C |
|
|
(j) Fujiwara, Y. J.; Dixon, A.; Baran, P. S . J. Am. Chem. Soc. 2012, 134, 1494.
doi: 10.1039/B610213C |
|
|
(k) Xu, L.; Vicic, D. A . J. Am. Chem. Soc. 2016, 138, 2536.
doi: 10.1039/B610213C |
|
|
(l) Qi, Q.-Q.; Shen, Q.-L.; Lu, L . J. Am. Chem. Soc. 2012, 134, 6548.
doi: 10.1039/B610213C |
|
|
(m) Feng, Z.; Zhang, X . Org. Lett. 2016, 18, 44.
doi: 10.1039/B610213C |
|
|
(n) Ruan, Z.-X.; Zhang, S.-K.; Ackermann, L . Angew. Chem., Int. Ed. 2017, 56, 2045.
doi: 10.1039/B610213C |
|
| [8] |
(a) Blackburn, C. M.; England, D. A.; Kolkmann, F . J. Chem. Soc. Chem. Commun. 1981,930.
doi: 10.6023/A12090668 |
|
(b) Yang, Y.; You, Z.; Qing, F.-L . Acta Chim. Sinica. 2012, 70, 2323 (in Chinese).
doi: 10.6023/A12090668 |
|
|
( 杨义, 游正伟, 卿凤翎 , 化学学报, 2012, 70, 2323.)
doi: 10.6023/A12090668 |
|
|
(c) An, L.; Tong, F.-F.; Zhang, X . Acta Chim. Sinica. 2018, 76, 977 (in Chinese).
doi: 10.6023/A12090668 |
|
|
( 安伦, 童非非, 张新刚 , source>化学学报, 2018, 76, 977.)
doi: 10.6023/A12090668 |
|
| [9] |
(a) Meanwell, N. A . J. Med. Chem. 2011, 54, 2529.
doi: 10.1021/jm1013693 |
|
(b) Meanwell, N. A . J. Med. Chem. 2018, 61, 5822.
doi: 10.1021/jm1013693 |
|
| [10] | (a) Huang, B. N.; Huang, W. Y.; Hu, C. M . Acta Chim. Sinica. 1981, 39, (in Chinese) 481. |
| ( 黄炳南, 黄维垣, 胡昌明 , 化学学报, 1981, 39, 481.) | |
| (b) Huang, B. N.; Huang, W. Y.; Hu, C. M . J. Fluorine Chem. 1983, 23, 193. | |
| (c) Huang, B. N.; Huang, W. Y.; Wang, W . Acta Chim. Sinica. 1983, 41, 1193 (in Chinese) | |
| ( 黄维垣, 黄炳南, 王巍 , 化学学报, 1983, 41, 1193.) | |
| (d) Wu, F.-H.; Huang, B. N.; Huang, W. Y . Chin. J. Org. Chem. 1993, 13, 449 (in Chinese) | |
| ( 吴范宏, 黄炳南, 黄维垣 , 有机化学, 1993, 13, 449.) | |
| [11] |
Shi, S.-L.; Buchwald, S.-L . Angew. Chem. Int. Ed. 207, 129, 2077.
doi: 10.1002/ange.201611595 |
| [12] |
(a) Belhomme, M.-C.; Poisson, T.; Pannecoucke, X . J. Org. Chem. 2014, 79, 7205
doi: 10.1021/jo5010907 |
|
(b) Wang, L.-P.; Liu, H.-Y.; Li, F.-F.; Zhao, J.-Q.; Zhang, H.-Y.; Zhang, Y.-C . Adv. Synth. Catal. 2019, 361, 2354.
doi: 10.1021/jo5010907 |
|
| [13] |
(a) Ruan, Z.-X.; Zhang, S.-K.; Zhu, C.-J.; Ruth, P.-N.; Stalke, D.; Ackermann, L . Angew. Chem., Int. Ed. 2017, 129, 2077.
doi: 10.1002/ange.201611595 |
|
(b) Li, Z.-Y.; Li, L.; Li, Q.-L.; Jing, K.; Xu, H.; Wang, G.-W . Chem. Eur. J., 2017, 23, 3285.
doi: 10.1002/ange.201611595 |
|
|
(c) Yuan, C.-C.; Chen, X.-L.; Zhang, J.-Y.; Zhao, Y.-S . Org. Chem. Front. 2017, 4, 1867.
doi: 10.1002/ange.201611595 |
|
| [14] |
(a) Chen, Y.-Y.; Lu, L.-Q.; Yu, D.-G.; Zhu, C.-J.; Xiao, W.-J . Sci China Chem. 2019, 62, 24.
doi: 10.1007/s11426-018-9399-2 |
|
(b) Liu, Q.; Wu, L.-Z . Nat. Sci. Rev. 2017, 4, 359.
doi: 10.1007/s11426-018-9399-2 |
|
|
(d) Skubi, K. L.; Yoon, T. P . Nature 2014, 515, 45.
doi: 10.1007/s11426-018-9399-2 |
|
| [15] |
(a) Lin, Q.; Chu, L.; Qing, F . Chin. J. Chem. 2013, 31, 885.
doi: 10.1002/cjoc.v31.7 |
|
(b) Yu, X.; Xu, X.-H.; Qing, F . Org. Lett. 2016, 18, 5130.
doi: 10.1002/cjoc.v31.7 |
|
|
(c) Su, Y.-M.; Hou, Y.; Yin, F.; Xu, Y.-M.; Li, Y.; Zheng, X.; Wang, X . Org. Lett. 2014, 16, 2958.
doi: 10.1002/cjoc.v31.7 |
|
|
(d) Jung, J.; Kim, E.; You, Y.; Cho, E. J . Adv. Synth. Catal. 2014, 356, 2741.
doi: 10.1002/cjoc.v31.7 |
|
|
(e) McAtee, R.-C.; Beatty, J.-W.; McAtee, C.-C.; Stephenson, C. R. J . Org. Lett. 2018, 20, 3491.
doi: 10.1002/cjoc.v31.7 |
|
|
(f) Wang, L.; Wei, X.-J.; Lei, W.-L.; Chen, H.; Wu, L.-Z.; Liu, Q . Chem. Commun. 2014, 50, 15916.
doi: 10.1002/cjoc.v31.7 |
|
|
(g) Wang, L.; Wei, X.-J.; Jia, W.-L.; Zhong, J.-J.; Wu, L.-Z.; Liu, Q . Org. Lett. 2014, 16, 5842.
doi: 10.1002/cjoc.v31.7 |
|
| [16] |
(a) Paria, S.; Reiser, O . ChemCatChem. 2014, 6, 2477.
doi: 10.1002/cctc.201402237 |
|
(b) Reiser, O . Acc. Chem. Res. 2016, 49, 1990.
doi: 10.1002/cctc.201402237 |
|
|
(c) Hernandez-Perez, A. C.; Collins, S. K . Acc. Chem. Res. 2016, 49, 1557.
doi: 10.1002/cctc.201402237 |
|
|
(d) Cuttell, D. G.; Kuang, S.-M.; Fanwick, P. E.; McMillin, D. R.; Walton, R . J. Am. Chem. Soc. 2002, 124, 6.
doi: 10.1002/cctc.201402237 |
|
|
(e) McMillin, D. R.; McNett, K. M . Chem. Rev. 1998, 98, 1201.
doi: 10.1002/cctc.201402237 |
|
|
(f) Cuttell, D. G.; Kuang, S.-M.; Fanwick, P. E.; McMillin, D. R.; Walton, R. A . J. Am. Chem. Soc. 2002, 124, 6.
doi: 10.1002/cctc.201402237 |
|
| [17] |
(a) Huang, J.; Mara, M. W.; Stickrath, A. B.; Kokhan, O.; Harpham, M. R.; Haldrup, K.; Shelby, M. L.; Zhang, X.; Ruppert, R.; Sauvage, J.-P.; Chen, L. X . Dalton Trans. 2014, 43, 17615.
doi: 10.1039/C4DT02046D |
|
(b) Pirtsch, M.; Paria, S.; Matsuno, T.; Isobe, H. T.; Reiser, O . Chem. Eur. J. 2012, 18, 7336.
doi: 10.1039/C4DT02046D |
|
|
(c) Paria, S.; Pirtsch, M.; Kais, V.; Reiser, O . Synthesis. 2013, 45, 2689.
doi: 10.1039/C4DT02046D |
|
|
(d) Tang, X.-J.; Dolbier, W. R., Jr. . Angew. Chem., Int. Ed. 2015, 54, 4246.
doi: 10.1039/C4DT02046D |
|
|
(e) Bagal, D. B.; Kachkovskyi, G.; Knorn, M.; Rawner, T.; Bhanage, B. M.; Reiser, O . Angew. Chem., Int. Ed. 2015, 54, 6999.
doi: 10.1039/C4DT02046D |
|
|
(f) Fumagalli, G.; Rabet, P. T. G.; Boyd, S.; Greaney, M. F . Angew. Chem., Int. Ed. 2015, 54, 11481.
doi: 10.1039/C4DT02046D |
|
|
(g) Rabet, P. T. G.; Fumagalli, G.; Boyd, S.; Greaney, M. F . Org. Lett. 2016, 18, 1646.
doi: 10.1039/C4DT02046D |
|
|
(h) Hossain, A.; Engl, S.; Lutsker, E.; Reiser, O . ACS Catal. 2019, 9, 1103.
doi: 10.1039/C4DT02046D |
|
| [18] |
(a) Hernandez-Perez, A. C.; Vlassova, A.; Collins, S. K . Org. Lett. 2012, 14, 2988.
doi: 10.1021/ol300983b |
|
(b) Knorn, M.; Rawner, T.; Czerwieniec, R.; Reiser, O . ACS Catal. 2015, 5, 5186.
doi: 10.1021/ol300983b |
|
|
(c) Murat, A.-Z.; Hu, X.-L . Organometallics. 2018, 37, 3928.
doi: 10.1021/ol300983b |
|
|
(d) Brunner, F.; Graber, S.; Baumgartner, Y.; Haussinger, D.; Prescimone, A.; Constable, E. C.; Housecroft, C. E . Dalton Trans. 2017, 46, 6379.
doi: 10.1021/ol300983b |
|
|
(e) Nitelet, N.; Thevenet, D.; Schiavi, B.; Hardouin, C.; Fournier, J.; Tamion, R.; Pannecoucke, X.; Jubault, P.; Poisson, T . Chem. Eur. J. 2019, 25, 3262.
doi: 10.1021/ol300983b |
|
|
(f) Wang, B.; Shelar, D. P.; Han, X.-Z.; Li, T.-T.; Guan, X.-G . Chem. Eur. J. 2015, 21, 1184.
doi: 10.1021/ol300983b |
|
|
(g) Michelet, B.; Deldaele, C.; Kajouj, S.; Moucheron, C.; Evano, G . Org. Lett. 2017, 19, 3576.
doi: 10.1021/ol300983b |
|
|
(h) Lu, W.; Liu, K.; Chen, Y.; Fu, W.-F.; Che, C.-M . Chem. Eur. J. 2015, 21, 1184.
doi: 10.1021/ol300983b |
|
| [19] |
(a) Hernandez-Perez, A. C.; Collins, S. K . Angew. Chem. Int. Ed. 2013, 52, 12696.
doi: 10.1002/anie.201306920 |
|
(b) Hernandez-Perez, A. C.; Collins, S. K . Angew. Chem. Int. Ed. 2013, 125, 12928.
doi: 10.1002/anie.201306920 |
|
|
(c) Hernandez-Perez, A. C.; Vlassova, A.; Collins, S. K . Org. Lett. 2012, 14, 2988.
doi: 10.1002/anie.201306920 |
|
| [20] |
(a) Ahn, J. M.; Peters, J. C.; Fu, G. C . J. Am. Chem. Soc. 2017, 139, 18101.
doi: 10.1021/jacs.7b10907 |
|
(b) Zhao, W.; Wurz, R. P.; Peters, J. C.; Fu, G. C . J. Am. Chem. Soc. 2017, 139, 12153.
doi: 10.1021/jacs.7b10907 |
|
| [21] |
Wang, W.; Guo, M.-Z.; Qi, R.-P.; Shang, Q.-Y.; Liu, Q.; Wang, S.; Zhao, L.; Wang, R.; Xu, Z.-Q . Angew. Chem. Int. Ed. 2018, 57, 15841.
doi: 10.1002/anie.201809400 |
| [22] |
Zhang, B.; Daniliuc, C. G.; Studer, A . Angew. Chem., Int. Ed. 2013, 52, 10792.
doi: 10.1002/anie.201306082 |
| [1] | 王志强, 苏进展. 磷酸钴修饰Cu3V2O8/ZnO光阳极的动力学特性及光电化学水分解研究[J]. 化学学报, 2024, 82(1): 26-35. |
| [2] | 李永雪, 刘育. 基于两亲性杯[4]芳烃的超分子二级组装及其生物应用★[J]. 化学学报, 2023, 81(8): 928-936. |
| [3] | 鱼章龙, 李忠良, 杨昌江, 顾强帅, 刘心元. 铜催化的二醇类化合物对映选择性去对称化反应研究进展★[J]. 化学学报, 2023, 81(8): 955-966. |
| [4] | 刘嘉文, 林玮璜, 王惟嘉, 郭学益, 杨英. Cu1.94S-SnS纳米异质结的合成及其光催化降解研究[J]. 化学学报, 2023, 81(7): 725-734. |
| [5] | 马长顺, 金苇航, 童非, 顾睿锐, 曲大辉. 一种可单晶到单晶(SCSC)转变的新型柱[5]芳烃的合成及性质研究★[J]. 化学学报, 2023, 81(6): 572-576. |
| [6] | 李飞, 丁汇丽, 李超忠. 基于氟仿衍生的三氟甲基硼络合物参与的烯烃氢三氟甲基化反应[J]. 化学学报, 2023, 81(6): 577-581. |
| [7] | 徐袁利, 潘辉, 杨义, 左智伟. 连续流条件下蒽-铈协同催化的苄位碳氢键选择性氧化反应★[J]. 化学学报, 2023, 81(5): 435-440. |
| [8] | 刘健, 欧金花, 李泽平, 蒋婧怡, 梁荣涛, 张文杰, 刘开建, 韩瑜. 金属-有机骨架衍生的Co单原子高效催化硝基芳烃氢化还原[J]. 化学学报, 2023, 81(12): 1701-1707. |
| [9] | 孟庆端, 韩佳宏, 潘一骁, 郝伟, 范青华. C1-对称手性氮杂环卡宾(NHC)配体的不对称合成及其催化性能研究★[J]. 化学学报, 2023, 81(10): 1271-1279. |
| [10] | 邱孔茜, 李杰, 马浩文, 周伟, 蔡倩. 捕捉环加成反应中的有机亚铜中间体构筑氮杂环化合物研究进展[J]. 化学学报, 2023, 81(1): 42-63. |
| [11] | 贾亚辉, 李春生, 徐忠震, 刘伟, 高道伟, 陈国柱. 载体相变下Pt-TiO2 SMSI研究及其对CO催化性能的影响[J]. 化学学报, 2022, 80(9): 1289-1298. |
| [12] | 眭玉光, 周锦荣, 廖攀, 梁文杰, 徐海. 巨型给-受体分子开关化合物的合成及性质研究[J]. 化学学报, 2022, 80(8): 1061-1065. |
| [13] | 吴明港, 杨勇, 薛敏. 四氨基柱[5]芳烃二聚体的合成、结构及性质研究[J]. 化学学报, 2022, 80(8): 1057-1060. |
| [14] | 郑安妮, 金磊, 杨家强, 李威青, 王赵云, 杨防祖, 詹东平, 田中群. 聚合物材料表面化学镀铜的前处理研究进展[J]. 化学学报, 2022, 80(5): 659-667. |
| [15] | 徐清浩, 魏立谱, 张震, 肖斌. 铜促进的锗亲电试剂与烷基溴合成四烷基锗※[J]. 化学学报, 2022, 80(4): 428-431. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||