化学学报 ›› 2021, Vol. 79 ›› Issue (2): 176-179.DOI: 10.6023/A20110520 上一篇 下一篇
研究通讯
周波1, 梁仁校1, 曹中艳1, 周平海1, 贾义霞1,*( )
)
投稿日期:2020-11-14
发布日期:2020-12-15
通讯作者:
贾义霞
作者简介:基金资助:
Bo Zhou1, Renxiao Liang1, Zhongyan Cao1, Pinghai Zhou1, Yixia Jia1,*( )
)
Received:2020-11-14
Published:2020-12-15
Contact:
Yixia Jia
Supported by:文章分享
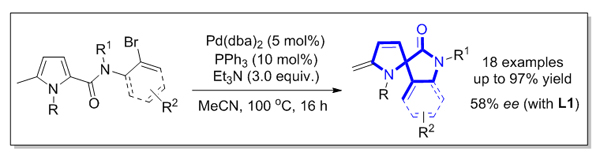
基于迁移插入策略, 研究了钯催化吡咯环内共轭双键的分子内Heck反应. 在温和的反应条件下, 以良好至优异的收率合成了一系列含有二氢吡咯及2-吲哚酮结构的螺杂环化合物. 同时, 以八氢联萘酚衍生的亚磷酰胺为手性配体, 初步探索该对映选择性反应, 获得中等水平的对映体过量值.
周波, 梁仁校, 曹中艳, 周平海, 贾义霞. 钯催化吡咯环内共轭双键的Heck反应[J]. 化学学报, 2021, 79(2): 176-179.
Bo Zhou, Renxiao Liang, Zhongyan Cao, Pinghai Zhou, Yixia Jia. Palladium-Catalyzed Heck Reaction of Endocyclic Conjugated C=C Bonds of Pyrroles[J]. Acta Chimica Sinica, 2021, 79(2): 176-179.
| Entry | Solvent | Ligand | Base | Yield/% |
|---|---|---|---|---|
| 1 | DMF | PPh3 | K2CO3 | 63 |
| 2 | toluene | PPh3 | K2CO3 | 88 |
| 3 | THF | PPh3 | K2CO3 | 90 |
| 4 | MeCN | PPh3 | K2CO3 | 92 |
| 5 | CHCl3 | PPh3 | K2CO3 | n.r. |
| 6 | MeCN | PCy3·HBF4 | K2CO3 | 90 |
| 7 | MeCN | P(O n Bu)3 | K2CO3 | 89 |
| 8 b | MeCN | dppe | K2CO3 | 17 |
| 9 b | MeCN | BINAP | K2CO3 | 20 |
| 10 | MeCN | PPh3 | Na2CO3 | 93 |
| 11 | MeCN | PPh3 | Cs2CO3 | 85 |
| 12 | MeCN | PPh3 | KF | 80 |
| 13 | MeCN | PPh3 | K3PO4 | 93 |
| 14 | MeCN | PPh3 | CH3CO2Na | 92 |
| 15 | MeCN | PPh3 | DBU | 76 |
| 16 | MeCN | PPh3 | Et3N | 97 |
| 17 c | MeCN | PPh3 | Et3N | 95 |
| 18 d | MeCN | PPh3 | Et3N | 51 |
| Entry | Solvent | Ligand | Base | Yield/% |
|---|---|---|---|---|
| 1 | DMF | PPh3 | K2CO3 | 63 |
| 2 | toluene | PPh3 | K2CO3 | 88 |
| 3 | THF | PPh3 | K2CO3 | 90 |
| 4 | MeCN | PPh3 | K2CO3 | 92 |
| 5 | CHCl3 | PPh3 | K2CO3 | n.r. |
| 6 | MeCN | PCy3·HBF4 | K2CO3 | 90 |
| 7 | MeCN | P(O n Bu)3 | K2CO3 | 89 |
| 8 b | MeCN | dppe | K2CO3 | 17 |
| 9 b | MeCN | BINAP | K2CO3 | 20 |
| 10 | MeCN | PPh3 | Na2CO3 | 93 |
| 11 | MeCN | PPh3 | Cs2CO3 | 85 |
| 12 | MeCN | PPh3 | KF | 80 |
| 13 | MeCN | PPh3 | K3PO4 | 93 |
| 14 | MeCN | PPh3 | CH3CO2Na | 92 |
| 15 | MeCN | PPh3 | DBU | 76 |
| 16 | MeCN | PPh3 | Et3N | 97 |
| 17 c | MeCN | PPh3 | Et3N | 95 |
| 18 d | MeCN | PPh3 | Et3N | 51 |
| [1] |
(a) Marti C. ; Carreira E.M. Eur. J. Org. Chem. 2003, 2003, 2209.
|
|
(b) Trost B.M. ; Brennan M.K. Synthesis 2009, 2009, 3003.
|
|
|
(c) Hong L. ; Wang R. Adv. Synth. Catal. 2013, 355, 1023.
|
|
|
(d) Saraswat P. ; Jeyabalan G. ; Hassan M.Z. ; Rahman M.U. ; Nyola N.K. Synth. Commun. 2016, 46, 1643.
|
|
|
(e) Xu P.-W. ; Yu J.-S. ; Chen C. ; Cao Z.-Y. ; Zhou F. ; Zhou J. ACS Catal. 2019, 9, 1820.
|
|
| [2] |
(a) Ali M.A. ; Ismail R. ; Choon T.S. ; Yoon Y.K. ; Wei A.C. ; Pandian S. ; Kumar R.S. ; Osman H. ; Manogaran E. Bioorg. Med. Chem. Lett. 2010, 20, 7064.
|
|
(b) George R.F. ; Ismail N.S.M. ; Stawinski J. ; Girgis A.S. Eur. J. Med. Chem. 2013, 68, 339.
|
|
|
(c) Arun Y. ; Bhaskar G. ; Balachandran C. ; Ignacimuthu S. ; Perumal P.T. Bioorg. Med. Chem. Lett. 2013, 23, 1839.
|
|
|
(d) Wu G. ; Ouyang L. ; Liu J. ; Zeng S. ; Huang W. ; Han B. ; Wu F. ; He G. ; Xiang M. Mol. Diversity 2013, 17, 271.
|
|
|
(e) Kumar A. ; Gupta G. ; Bishnoi A.K. ; Saxena R. ; Saini K.S. ; Konwar R. ; Kumar S. ; Dwivedi A. Bioorg. Med. Chem. 2015, 23, 839.
|
|
|
(f) Kathirvelan D. ; Haribabu J. ; Reddy B.S.R. ; Balachandran C. ; Duraipandiyan V. Bioorg. Med. Chem. Lett. 2015, 25, 389.
|
|
| [3] |
(a) Pape A.R. ; Kaliappan K.P. ; Kündig E.P. Chem. Rev. 2000, 100, 2917.
|
|
(b) López Ortiz, F. ; Iglesias, M.J. ; Fernández, I. ; Andújar Sánchez, C.M.; Gómez, G.R. Chem. Rev. 2007, 107, 1580.
|
|
|
(c) Roche S.P. ; Porco J.A.Jr. Angew. Chem., Int. Ed. 2011, 50, 4068.
|
|
|
(d) Zhuo C.-X. ; Zhang W. ; You S.-L. Angew. Chem., Int. Ed. 2012, 51, 12662.
|
|
|
(e) Zhuo C.-X. ; Zheng C. ; You S.-L. Acc. Chem. Res. 2014, 47, 2558.
|
|
|
(f) Wu W.-T. ; Zhang L. ; You S.-L. Chem. Soc. Rev. 2016, 45, 1570.
|
|
|
(g) Zheng C. ; You S.-L. Chem. 2016, 1, 830.
|
|
|
(h) Wu W.-T. ; Zhang L. ; You S.-L. Acta Chim. Sinica 2017, 75, 419. (in Chinese)
|
|
|
吴文挺, 张立明, 游书力, 化学学报, 2017, 75, 419.
|
|
|
(i) Wertjes W.C. ; Southgate E.H. ; Sarlah D. Chem. Soc. Rev. 2018, 47, 7996.
|
|
|
(j) Zhu M. ; Zhang X. ; You S.-L. Chem. J. Chin. Univ. 2020, 41, 1407. (in Chinese)
|
|
|
朱敏, 张霄, 游书力, 高学校化学学报, 2020, 41, 1407.
|
|
| [4] |
Zeidan N. ; Lautens M. Synthesis 2019, 51, 4137.
|
| [5] |
(a) Zhao L. ; Li Z. ; Chang L. ; Xu J. ; Yao H. ; Wu X. Org. Lett. 2012, 14, 2066.
|
|
(b) Shen C. ; Liu R.-R. ; Fan R.-J. ; Li Y.-L. ; Xu T.-F. ; Gao J.-R. ; Jia Y.-X. J. Am. Chem. Soc. 2015, 137, 4936.
|
|
|
(c) Petrone D.A. ; Yen A. ; Zeidan N. ; Lautens M. Org. Lett. 2015, 17, 4838.
|
|
|
(d) Petrone D.A. ; Kondo M. ; Zeidan N. ; Lautens M. Chem. Eur. J. 2016, 22, 5684.
|
|
|
(e) Chen S. ; Wu X.-X. ; Wang J. ; Hao X.-H. ; Xia Y. ; Shen Y. ; Jing H. ; Liang Y.-M. Org. Lett. 2016, 18, 4016.
|
|
|
(f) Liu R.-R. ; Xu T.-F. ; Wang Y.-G. ; Xiang B. ; Gao J.-R. ; Jia Y.-X. Chem. Commun. 2016, 52, 13664.
|
|
|
(g) Gao S. ; Yang C. ; Huang Y. ; Zhao L. ; Wu X. ; Yao H. ; Lin A. Org. Biomol. Chem. 2016, 14, 840.
|
|
|
(h) Douki K. ; Ono H. ; Taniguchi T. ; Shimokawa J. ; Kitamura M. ; Fukuyama T. J. Am. Chem. Soc. 2016, 138, 14578.
|
|
|
(i) Wang Y. ; Liu R. ; Gao J. ; Jia Y. Chin. J. Org. Chem. 2017, 37, 691. (in Chinese)
|
|
|
王永刚, 刘人荣, 高建荣, 贾义霞, 有机化学, 2017, 37, 691.
|
|
|
(j) Qin X. ; Lee M.W.Y. ; Zhou J.S. Angew. Chem., Int. Ed. 2017, 56, 12723.
|
|
|
(k) Liu R.-R. ; Wang Y.-G. ; Li Y.-L. ; Huang B.-B. ; Liang R.-X. ; Jia Y.-X. Angew. Chem., Int. Ed. 2017, 56, 7475.
|
|
|
(l) Xu X. ; Liu J. ; Lu L. ; Wang F. ; Yin B. Chem. Commun. 2017, 53, 7796.
|
|
|
(m) Liu R.-R. ; Xu Y. ; Liang R.-X. ; Xiang B. ; Xie H.-J. ; Gao J.-R. ; Jia Y.-X. Org. Biomol. Chem. 2017, 15, 2711.
|
|
|
(n) Liang R.-X. ; Yang R.-Z. ; Liu R.-R. ; Jia Y.-X. Org. Chem. Front. 2018, 5, 1840.
|
|
|
(o) Zeidan N. ; Beisel T. ; Ross R. ; Lautens M. Org. Lett. 2018, 20, 7332.
|
|
|
(p) Li X. ; Zhou B. ; Yang R.-Z. ; Yang F.-M. ; Liang R.-X. ; Liu R.-R. ; Jia Y.-X. J. Am. Chem. Soc. 2018, 140, 13945.
|
|
|
(q) Wang H. ; Wu X.-F. Org. Lett. 2019, 21, 5264.
|
|
|
(r) Liang R.-X. ; Wang K. ; Wu Q. ; Sheng W.-J. ; Jia Y.-X. Organometallics 2019, 38, 3927.
|
|
|
(s) Shen C. ; Zeidan N. ; Wu Q. ; Breuers C.B.J. ; Liu R.-R. ; Jia Y.-X. ; Lautens M. Chem. Sci. 2019, 10, 3118.
|
|
|
(t) Marchese A.D. ; Lind F. ; Mahon Á. E. ; Yoon H. ; Lautens M . Angew. Chem., Int. Ed. 2019, 58, 5095.
|
|
|
(u) Huang L. ; Cai Y. ; Zhang H.-J. ; Zheng C. ; Dai L.-X. ; You S.-L. CCS Chem. 2019, 1, 106.
|
|
|
(v) Zhang Z. ; Zhang B.-S. ; Li K.-L. ; An Y. ; Liu C. ; Gou X.-Y. ; Liang Y.-M. J. Org. Chem. 2020, 85, 7817.
|
|
|
(w) Yang P. ; Xu R.-Q. ; Zheng C. ; You S.-L. Chin. J. Chem. 2020, 38, 235.
|
|
|
(x) Yang P. Chin. J. Chem. 2020, 38, 525. (y) Zhu, M.; Huang, X.-L.; Xu, H.; Zhang, X.; Zheng, C.; You, S.-L. CCS Chem. 2020, 2, 652.
|
|
| [6] |
(a) Liu J. ; Peng H. ; Lu L. ; Xu X. ; Jiang H. ; Yin B. Org. Lett. 2016, 18, 6440.
|
|
(b) Liu J. ; Xu X. ; Li J. ; Liu B. ; Jiang H. ; Yin B. Chem. Commun. 2016, 52, 9550.
|
|
|
(c) Liu J. ; Peng H. ; Yang Y. ; Jiang H. ; Yin B. J. Org. Chem. 2016, 81, 9695.
|
|
| [7] |
Yao T. ; Zhang F. ; Zhang J. ; Liu L. Org. Lett. 2020, 22, 5063.
|
| [8] |
(a) Zuo Z. ; Wang H. ; Diao Y. ; Ge Y. ; Liu J. ; Luan X. ACS Catal. 2018, 8, 11029.
|
|
(b) Liao X. ; Wang D. ; Huang Y. ; Yang Y. ; You J. Org. Lett. 2019, 21, 1152.
|
|
|
(c) Yang P. ; Zheng C. ; Nie Y.-H. ; You S.-L. Chem. Sci. 2020, 11, 6830.
|
|
|
(d) Zhou B. ; Wang H. ; Cao Z.-Y. ; Zhu J.-W. ; Liang R.-X. ; Hong X. ; Jia Y.-X. Nat. Commun. 2020, 11, 4380.
|
|
|
(e) Chen M. ; Wang X. ; Ren Z.-H. ; Guan Z.-H. CCS Chem. doi.org/10.31635/ccschem.020.202000596.
|
|
| [9] |
Huang J. ; Fu R. ; Jing L. ; Qin D. ; Huang K. ; Wang W . Chin. J. Org. Chem. 2019, 39, 456. (in Chinese)
|
|
黄锦, 付荣辉, 敬林海, 秦大斌, 黄昆, 汪伟, 有机化学, 2019, 39, 456.”
|
|
| [10] |
(a) Ren H. ; Li Z. ; Knochel P. Chem. - Asian J. 2007, 2, 416.
|
|
(b) Yang P. ; You S.-L. Org. Lett. 2018, 20, 7684.
|
| [1] | 张大伟, 赵海洋, 冯笑甜, 顾玉诚, 张新刚. 钯催化下杂芳基溴代物与偕二氟烯丙基硼试剂的交叉偶联反应[J]. 化学学报, 2024, 82(2): 105-109. |
| [2] | 高炜洋, 邓伟超, 高扬, 梁仁校, 贾义霞. 吲哚分子间不对称去芳构化氧化Heck反应[J]. 化学学报, 2024, 82(1): 1-4. |
| [3] | 黄涎廷, 韩洪亮, 肖婧, 王帆, 柳忠全. I2O5/KSCN介导的炔烃碘硫氰化反应[J]. 化学学报, 2024, 82(1): 5-8. |
| [4] | 吕鑫, 吴仪, 张勃然, 郭炜. 过氧化氢激活型近红外氟硼二吡咯光敏剂的设计、合成及光动力治疗研究[J]. 化学学报, 2023, 81(4): 359-370. |
| [5] | 黄艳琴, 栗丽君, 杨书培, 张瑞, 刘兴奋, 范曲立, 黄维. HA-AuNPs/FDF用于透明质酸酶的高灵敏检测、肿瘤靶向细胞荧光成像和光疗[J]. 化学学报, 2023, 81(12): 1687-1694. |
| [6] | 韩叶强, 史炳锋. 钯(II)催化不对称C(sp3)—H键官能团化研究进展★[J]. 化学学报, 2023, 81(11): 1522-1540. |
| [7] | 孟庆端, 韩佳宏, 潘一骁, 郝伟, 范青华. C1-对称手性氮杂环卡宾(NHC)配体的不对称合成及其催化性能研究★[J]. 化学学报, 2023, 81(10): 1271-1279. |
| [8] | 田小茂, 林悦群, 朱菡, 黄超, 朱必学. 手性单Schiff碱大环对青霉胺对映体识别研究[J]. 化学学报, 2023, 81(1): 20-28. |
| [9] | 刘巴蒂, 王承俊, 钱鹰. 噻吩基氟硼二吡咯近红外光敏染料的合成、双光子荧光成像及光动力治疗研究[J]. 化学学报, 2022, 80(8): 1071-1083. |
| [10] | 葛懿修, 邱早早, 谢作伟. 钯催化硼-碳和硼-杂原子键构建一锅法合成双官能团化邻-碳硼烷※[J]. 化学学报, 2022, 80(4): 432-437. |
| [11] | 郑龙, 王丽佳, 唐勇. 吲哚-环丙烷的分子内开环反应※[J]. 化学学报, 2022, 80(3): 255-258. |
| [12] | 徐云芳, 李阳, 付梓桐, 林绍艳, 祝洁, 吴磊. 钯催化选择性构筑(Z)-[3]戟烯反应研究[J]. 化学学报, 2022, 80(10): 1369-1375. |
| [13] | 杨普苏, 刘晨旭, 张文文, 游书力. 铱催化中氮茚衍生物的Friedel-Crafts类型不对称烯丙基取代反应[J]. 化学学报, 2021, 79(6): 742-746. |
| [14] | 邓卓基, 欧阳溢凡, 敖运林, 蔡倩. 铜催化不对称去对称化分子内烯基C—N偶联反应[J]. 化学学报, 2021, 79(5): 649-652. |
| [15] | 张荣华, 许冰, 张展鸣, 张俊良. Ming-Phos/铜催化的亚甲胺叶立德与硝基烯烃的不对称[3+2]环加成反应[J]. 化学学报, 2020, 78(3): 245-249. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||