化学学报 ›› 2021, Vol. 79 ›› Issue (7): 885-902.DOI: 10.6023/A21030126 上一篇 下一篇
综述
投稿日期:2021-03-31
发布日期:2021-05-11
通讯作者:
卢章辉
作者简介: |
张安琪, 1997年出生, 2019年本科毕业于巢湖学院, 随后加入江西师范大学卢章辉教授课题组攻读硕士学位, 主要研究方向为纳米材料的可控合成及其在能源与催化领域的应用. |
 |
姚淇露, 江西师范大学先进材料研究院助理研究员. 2017年于江西师范大学获得理学博士学位. 毕业后留校进入先进材料研究院工作. 主要研究方向为纳米材料的可控合成及其在能源与催化领域的应用. 以第一作者或通讯作者在国内外知名期刊发表SCI收录论文20余篇; 获授权发明专利5项; 获江西省自然科学奖一等奖(第二完成人). |
 |
卢章辉, 江西师范大学化学化工学院教授/博导, 江西省赣鄱英才555工程领军人才. 2011年于日本国立神户大学获得博士学位, 2008年10月至2011年9月, 在日本产业技术综合研究所从事研究, 2011年10月至今在江西师范大学工作. 主要从事能源催化研究, 在国内外知名期刊发表SCI收录论文110篇, 被引4500余次, 获江西省自然科学奖一等奖(第一完成人). |
基金资助:
Anqi Zhang, Qilu Yao, Zhang-Hui Lu( )
)
Received:2021-03-31
Published:2021-05-11
Contact:
Zhang-Hui Lu
Supported by:文章分享

氢气作为21世纪最具发展前景的清洁能源, 一直备受关注. 寻找安全高效的储氢材料以转型到氢能社会是当前面临的最大挑战之一. 水合肼(N2H4•H2O)具有高含氢量(w=8.0%), 完全分解产氢副产物仅为氮气和水, 被视为一种极具应用潜力的液相化学储氢材料. 开发高效、高选择性的催化剂以催化水合肼完全分解, 是研究水合肼分解产氢的关键. 本综述总结了水合肼分解产氢催化剂的设计、合成及其催化性能. 简要分析了肼分解的机理. 此外, 讨论了提高水合肼分解产氢催化剂的选择性和活性的策略, 比如添加强碱助剂/碱性载体、形成合金、降低金属催化剂的结晶度、减小粒子尺寸、以及增强金属与载体相互作用. 本研究进展可以为设计合成具有更高活性的氮基氢化物产氢催化剂提供指导和思路.
张安琪, 姚淇露, 卢章辉. 水合肼分解产氢催化剂研究进展[J]. 化学学报, 2021, 79(7): 885-902.
Anqi Zhang, Qilu Yao, Zhang-Hui Lu. Recent Progress on Catalysts for Hydrogen Evolution from Decomposition of Hydrous Hydrazine[J]. Acta Chimica Sinica, 2021, 79(7): 885-902.
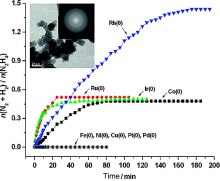

| Catalyst | T/℃ | TOF/h-1 | Hydrogen selectivity/% | Ref. |
|---|---|---|---|---|
| Pt | 25 | — | 0 | [ |
| Pd | 25 | — | 0 | [ |
| Cu | 25 | — | 0 | [ |
| Fe | 25 | — | 0 | [ |
| Ni | 25 | — | 0 | [ |
| Co | 25 | — | 7 | [ |
| Ru | 25 | — | 7 | [ |
| Ir | 25 | — | 7 | [ |
| Rh | 25 | — | 43.8 | [ |
| Ni-Al2O3-HT | 30 | 2.0a | 93 | [ |
| Ni/Al2O3-IMP | 30 | 2.86a,b | 66 | [ |
| Ni-CeO2 | 30 | 51.6 | 99 | [ |
| Raney Ni-300 | 30 | 162a,b | 99 | [ |
| Ni/CeO2 | 50 | 34 | 100 | [ |
| Ni@TNTs | 60 | 96a | 100 | [ |
| Rh | 70 | — | 34 | [ |
| Ir/Al2O3 | >200 | — | 100 | [ |
| Ir/γ-Al2O3 | >200 | — | 100 | [ |
| Ni-CeO2@SiO2 | 70 | 219.5 | 100 | [ |
| Catalyst | T/℃ | TOF/h-1 | Hydrogen selectivity/% | Ref. |
|---|---|---|---|---|
| Pt | 25 | — | 0 | [ |
| Pd | 25 | — | 0 | [ |
| Cu | 25 | — | 0 | [ |
| Fe | 25 | — | 0 | [ |
| Ni | 25 | — | 0 | [ |
| Co | 25 | — | 7 | [ |
| Ru | 25 | — | 7 | [ |
| Ir | 25 | — | 7 | [ |
| Rh | 25 | — | 43.8 | [ |
| Ni-Al2O3-HT | 30 | 2.0a | 93 | [ |
| Ni/Al2O3-IMP | 30 | 2.86a,b | 66 | [ |
| Ni-CeO2 | 30 | 51.6 | 99 | [ |
| Raney Ni-300 | 30 | 162a,b | 99 | [ |
| Ni/CeO2 | 50 | 34 | 100 | [ |
| Ni@TNTs | 60 | 96a | 100 | [ |
| Rh | 70 | — | 34 | [ |
| Ir/Al2O3 | >200 | — | 100 | [ |
| Ir/γ-Al2O3 | >200 | — | 100 | [ |
| Ni-CeO2@SiO2 | 70 | 219.5 | 100 | [ |
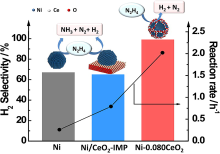
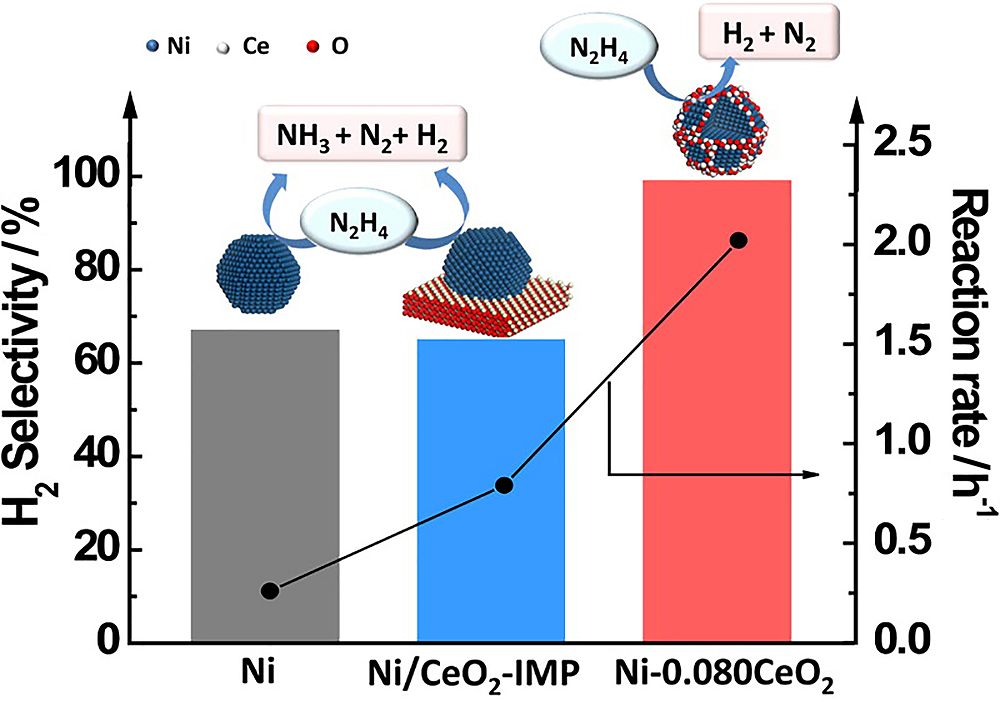
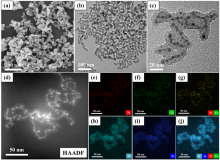


| Catalyst | T/℃ | TOF/h-1 | Hydrogen selectivity/% | Ref. |
|---|---|---|---|---|
| Ni0.6Pd0.4 | 25 | — | 7 | [ |
| Ni30Fe30Pd40 | 25 | 4.0b | 100 | [ |
| Ni0.95Ir0.05-CTAB | 25 | 3.08b | 100 | [ |
| Ni0.6Pd0.4 | 25 | 20a,b | 100 | [ |
| NiRh@rGO | 25 | 24.5b | 100 | [ |
| CoIr0.081/γ-Al2O3 | 25 | 27.76 | 100 | [ |
| Ni0.9Pt0.1/Ce2O3 | 25 | 28.1a | 100 | [ |
| NiPtRh/La2O3 | 25 | 45.9a | 100 | [ |
| Rh/Ni@SiO2 | 25 | 66a | 99.4 | [ |
| Ni64Pt36/MIL-96 | 25 | 114.3a | 100 | [ |
| (Ni3Pt7)0.5-(MnO x)0.5/NPC-900 | 25 | 120a | 100 | [ |
| NiPt/NH2-MIL-101 | 25 | 137a | 100 | [ |
| Ni60Pt40-SF-I | 25 | 150a | 100 | [ |
| Co0.65Pt0.30(CeO x)0.05 | 25 | 194.8a | 72.1 | [ |
| Ni0.7Pt0.3P/rGO | 25 | 224a | 100 | [ |
| NiIr0.059/Al2O3 | 30 | 12.4a | 99 | [ |
| Ni0.2Rh0.8@CeO x/rGO | 30 | 36.4b | 100 | [ |
| Ni0.9Pt0.1/MIL-101 | 30 | 140a | 100 | [ |
| Ni45Rh55/Ce(OH)CO3 | 30 | 150a | 100 | [ |
| Ni40Pt60-CNDs | 30 | 170a | 100 | [ |
| Ni60Pt40/CeO2 | 30 | 293 | 100 | [ |
| Ni0.58Pt0.42/grapheme | 30 | 434a | 100 | [ |
| CoPt/La(OH)3 | 30 | 734.2a | 100 | [ |
| Ni0.4Pt0.6/PDA-rGO | 30 | 903a | 100 | [ |
| Ni0.9Rh0.1 | 50 | 0.045b | 100 | [ |
| Ni0.99Pt0.01 | 50 | 5.7b | 100 | [ |
| Ni0.6Pd0.4 | 50 | 6.3b | 82 | [ |
| NiRh@ZIF-8 | 50 | 140a | 100 | [ |
| Ni3Rh7/NPC-900 | 50 | 156a | 100 | [ |
| NiPt/C | 50 | 210a | 100 | [ |
| Ni@Ni-Pt/La2O3 | 50 | 312a | 100 | [ |
| Ni42Rh58@MIL-101 | 50 | 344a | 100 | [ |
| Ni0.2Rh0.8/MIL-101 | 50 | 428.6 | 100 | [ |
| NiIr/MIL-101 | 50 | 464a | 100 | [ |
| RhNiP/rGO | 50 | 471a | 100 | [ |
| NiIr/La2O2CO3 | 50 | 487a | 100 | [ |
| Rh0.5(MoO x)0.5 | 50 | 750a | 100 | [ |
| Ni0.6Pt0.4-MoO x | 50 | 822a | 100 | [ |
| Rh92.6P7.4/rGO | 50 | 843.9a | 100 | [ |
| Ni0.2Rh0.8/Mxene | 50 | 857a | 100 | [ |
| NiPt/DT-Ti3C2T x | 50 | 1220a | 100 | [ |
| Ni0.4Pt0.6@ZrO2/C/rGO | 50 | 1920a | 100 | [ |
| CoPt/La(OH)3 | 50 | 2400a | 100 | [ |
| Catalyst | T/℃ | TOF/h-1 | Hydrogen selectivity/% | Ref. |
|---|---|---|---|---|
| Ni0.6Pd0.4 | 25 | — | 7 | [ |
| Ni30Fe30Pd40 | 25 | 4.0b | 100 | [ |
| Ni0.95Ir0.05-CTAB | 25 | 3.08b | 100 | [ |
| Ni0.6Pd0.4 | 25 | 20a,b | 100 | [ |
| NiRh@rGO | 25 | 24.5b | 100 | [ |
| CoIr0.081/γ-Al2O3 | 25 | 27.76 | 100 | [ |
| Ni0.9Pt0.1/Ce2O3 | 25 | 28.1a | 100 | [ |
| NiPtRh/La2O3 | 25 | 45.9a | 100 | [ |
| Rh/Ni@SiO2 | 25 | 66a | 99.4 | [ |
| Ni64Pt36/MIL-96 | 25 | 114.3a | 100 | [ |
| (Ni3Pt7)0.5-(MnO x)0.5/NPC-900 | 25 | 120a | 100 | [ |
| NiPt/NH2-MIL-101 | 25 | 137a | 100 | [ |
| Ni60Pt40-SF-I | 25 | 150a | 100 | [ |
| Co0.65Pt0.30(CeO x)0.05 | 25 | 194.8a | 72.1 | [ |
| Ni0.7Pt0.3P/rGO | 25 | 224a | 100 | [ |
| NiIr0.059/Al2O3 | 30 | 12.4a | 99 | [ |
| Ni0.2Rh0.8@CeO x/rGO | 30 | 36.4b | 100 | [ |
| Ni0.9Pt0.1/MIL-101 | 30 | 140a | 100 | [ |
| Ni45Rh55/Ce(OH)CO3 | 30 | 150a | 100 | [ |
| Ni40Pt60-CNDs | 30 | 170a | 100 | [ |
| Ni60Pt40/CeO2 | 30 | 293 | 100 | [ |
| Ni0.58Pt0.42/grapheme | 30 | 434a | 100 | [ |
| CoPt/La(OH)3 | 30 | 734.2a | 100 | [ |
| Ni0.4Pt0.6/PDA-rGO | 30 | 903a | 100 | [ |
| Ni0.9Rh0.1 | 50 | 0.045b | 100 | [ |
| Ni0.99Pt0.01 | 50 | 5.7b | 100 | [ |
| Ni0.6Pd0.4 | 50 | 6.3b | 82 | [ |
| NiRh@ZIF-8 | 50 | 140a | 100 | [ |
| Ni3Rh7/NPC-900 | 50 | 156a | 100 | [ |
| NiPt/C | 50 | 210a | 100 | [ |
| Ni@Ni-Pt/La2O3 | 50 | 312a | 100 | [ |
| Ni42Rh58@MIL-101 | 50 | 344a | 100 | [ |
| Ni0.2Rh0.8/MIL-101 | 50 | 428.6 | 100 | [ |
| NiIr/MIL-101 | 50 | 464a | 100 | [ |
| RhNiP/rGO | 50 | 471a | 100 | [ |
| NiIr/La2O2CO3 | 50 | 487a | 100 | [ |
| Rh0.5(MoO x)0.5 | 50 | 750a | 100 | [ |
| Ni0.6Pt0.4-MoO x | 50 | 822a | 100 | [ |
| Rh92.6P7.4/rGO | 50 | 843.9a | 100 | [ |
| Ni0.2Rh0.8/Mxene | 50 | 857a | 100 | [ |
| NiPt/DT-Ti3C2T x | 50 | 1220a | 100 | [ |
| Ni0.4Pt0.6@ZrO2/C/rGO | 50 | 1920a | 100 | [ |
| CoPt/La(OH)3 | 50 | 2400a | 100 | [ |
| Catalyst | T/℃ | TOF/h-1 | Hydrogen selectivity/% | Ref. |
|---|---|---|---|---|
| NiCoP1.5/SiO2 | 25 | 1.71 | 100 | [ |
| NiCo/NiO-CoO x | 25 | 5.49 | 100 | [ |
| Ni1.5Fe/(MgO)2.5 | 25 | 24b | 99 | [ |
| Co4N-Al2O3 (HT) | 50 | — | 100 | [ |
| NiFe/CeO2 | 50 | 5.73 | 99 | [ |
| Ni0.6Fe0.4Mo | 50 | 28.8 | 100 | [ |
| CuNiMo | 50 | 38.7 | 100 | [ |
| CuNi/CeO2 | 50 | 1450a | 100 | [ |
| Ni3Fe-(CeO x)0.15/rGO | 55 | 56.8a | 100 | [ |
| Cu0.5Ni0.5/MCNS | 60 | 21.8 | 100 | [ |
| NiFe | 70 | 6.3a,b | 100 | [ |
| Cu@Fe5Ni5 | 70 | 20b | 100 | [ |
| Ni4Mo@Cu2O | 70 | 71.4 | 100 | [ |
| NiFe/LaZrO2 | 70 | 100.3a | 100 | [ |
| NiFe/NdZrO2 | 70 | 103.7a | 100 | [ |
| NiFe/CeZrO2 | 70 | 119.2a | 100 | [ |
| Ni3Fe-(CeO x)0.15/rGO | 70 | 126.2a | 100 | [ |
| Ni0.9Fe0.1-Cr2O3 | 70 | 893.5a | 100 | [ |
| Catalyst | T/℃ | TOF/h-1 | Hydrogen selectivity/% | Ref. |
|---|---|---|---|---|
| NiCoP1.5/SiO2 | 25 | 1.71 | 100 | [ |
| NiCo/NiO-CoO x | 25 | 5.49 | 100 | [ |
| Ni1.5Fe/(MgO)2.5 | 25 | 24b | 99 | [ |
| Co4N-Al2O3 (HT) | 50 | — | 100 | [ |
| NiFe/CeO2 | 50 | 5.73 | 99 | [ |
| Ni0.6Fe0.4Mo | 50 | 28.8 | 100 | [ |
| CuNiMo | 50 | 38.7 | 100 | [ |
| CuNi/CeO2 | 50 | 1450a | 100 | [ |
| Ni3Fe-(CeO x)0.15/rGO | 55 | 56.8a | 100 | [ |
| Cu0.5Ni0.5/MCNS | 60 | 21.8 | 100 | [ |
| NiFe | 70 | 6.3a,b | 100 | [ |
| Cu@Fe5Ni5 | 70 | 20b | 100 | [ |
| Ni4Mo@Cu2O | 70 | 71.4 | 100 | [ |
| NiFe/LaZrO2 | 70 | 100.3a | 100 | [ |
| NiFe/NdZrO2 | 70 | 103.7a | 100 | [ |
| NiFe/CeZrO2 | 70 | 119.2a | 100 | [ |
| Ni3Fe-(CeO x)0.15/rGO | 70 | 126.2a | 100 | [ |
| Ni0.9Fe0.1-Cr2O3 | 70 | 893.5a | 100 | [ |





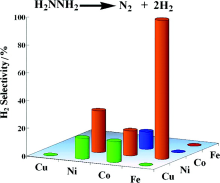
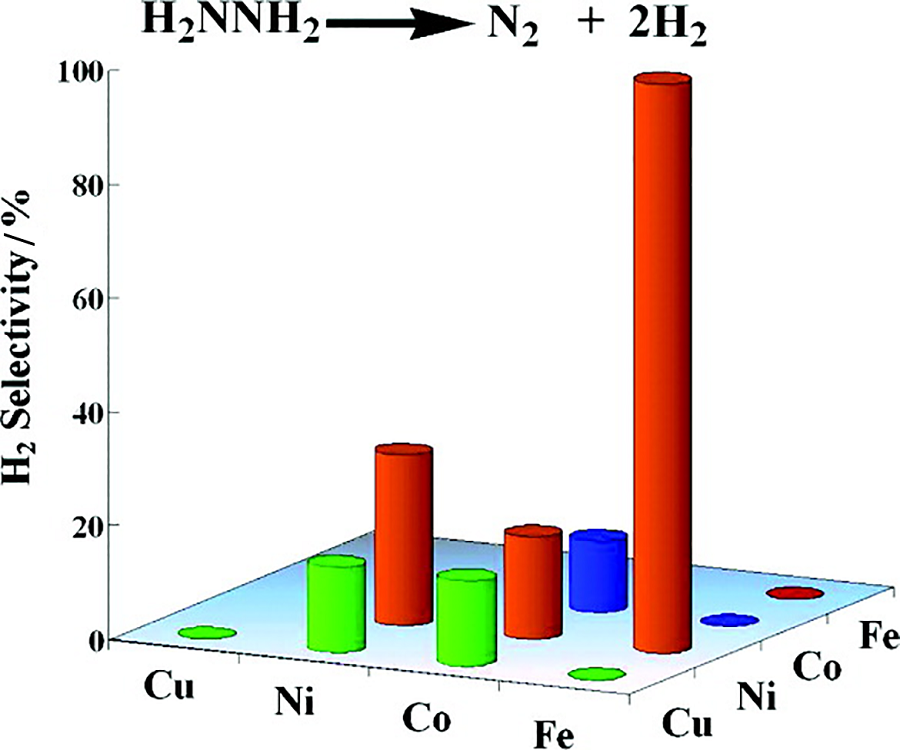
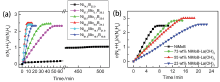
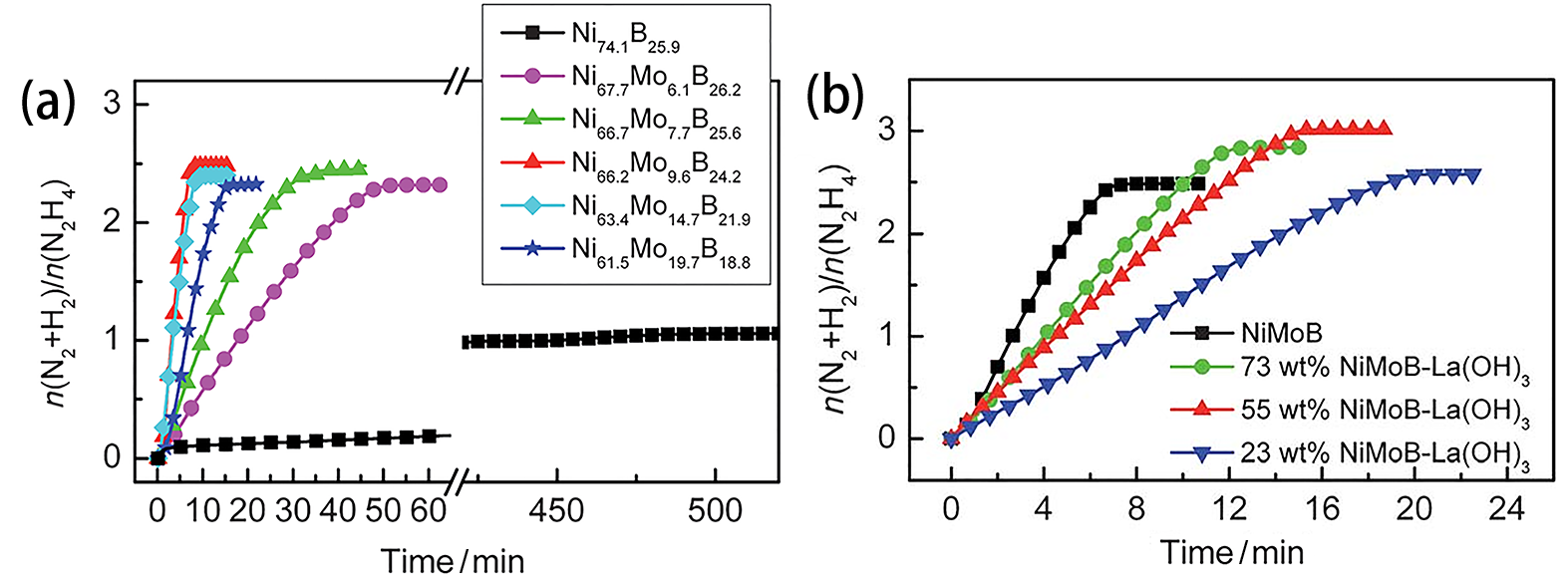
| [1] |
Gong, K.; Du, F.; Xia, Z. H.; Durstock, M.; Dai, L. M. Science 2009, 323, 760.
doi: 10.1126/science.1168049 |
| [2] |
Pan, Z. Y.; Tang, Z.; Zhan, Y. Z.; Sun, D. Tungsten 2020, 2, 390.
doi: 10.1007/s42864-020-00065-3 |
| [3] |
He, B.; Ren, Y. X.; Dai, T. J. Rare Metals 2019, 40, 219.
doi: 10.1007/s12598-019-01344-w |
| [4] |
Zhang, T. L.; Lu, Z. G.; Zhang, L. Y. Chin. Chem. Lett. 2020, 31, 3135.
doi: 10.1016/j.cclet.2020.07.010 |
| [5] |
Liang, Z. B.; Zhao, R.; Qiu, T. J.; Zou, R. Q.; Xu, Q. EnergyChem 2019, 1, 100001.
doi: 10.1016/j.enchem.2019.100001 |
| [6] |
Grochala, W.; Edwards, P. P. Chem. Rev. 2004, 104, 1283.
doi: 10.1021/cr030691s |
| [7] |
Schlapbach, L.; Züttel, A. Nature 2001, 414, 353.
doi: 10.1038/35104634 |
| [8] |
Chen, P.; Xiong, Z.; Luo, J.; Lin, J.; Tan, K. L. Nature 2002, 420, 302.
doi: 10.1038/nature01210 |
| [9] |
Li, X. R.; Yang, X. C.; Xue, H. G.; Pang, H.; Xu, Q. EnergyChem 2020, 2, 100027.
doi: 10.1016/j.enchem.2020.100027 |
| [10] |
Li, W. H.; Deng, W. H.; Wang, G-E.; Xu, G. EnergyChem 2020, 2, 100029.
doi: 10.1016/j.enchem.2020.100029 |
| [11] |
Zhang, X. T.; Chen, J. C.; Zhu, L.; Hao, S.; Jiang, D. M.; Xia, L. S. Appl. Chem. Ind. 2018, 47, 139. (in Chinese)
|
|
(张晓腾, 陈俊畅, 朱林, 郝帅, 蒋冬梅, 夏良树, 应用化工, 2018, 47, 139.)
|
|
| [12] |
Xue, S. F.; Li, Y. J.; Zheng, F. H.; Bian, X.; Wu, W. Y.; Yang, C. H. Rare Metals 2021, 40, 31.
doi: 10.1007/s12598-020-01594-z |
| [13] |
Cheng, Y.; Wu, X.; Xu, H. Sustain. Energy Fuels 2019, 3, 343.
doi: 10.1039/C8SE00538A |
| [14] |
Zhan, J. J.; Chen, Z. H. Mater. Rep. 2007, 62, 66. (in Chinese)
|
|
(湛建阶, 陈朝辉, 材料导报, 2007, 62, 66.)
|
|
| [15] |
Cho, S. J.; Lee, J.; Lee, Y. S.; Kim, D. P. Catal. Lett. 2006, 109, 181.
doi: 10.1007/s10562-006-0081-3 |
| [16] |
Jang, Y. B.; Kim, T. H.; Sun, M. H.; Lee, J.; Cho, S. J. Catal. Today 2009, 146, 196.
doi: 10.1016/j.cattod.2009.01.040 |
| [17] |
Singh, S. K.; Zhang, X.-B.; Xu, Q. J. Am. Chem. Soc. 2009, 131, 9894.
doi: 10.1021/ja903869y |
| [18] |
He, L.; Huang, Y.; Wang, A.; Wang, X.; Chen, X.; Delgado, J. J.; Zhang, T. Angew. Chem. Int. Ed. 2012, 51, 6191.
doi: 10.1002/anie.201201737 |
| [19] |
He, L.; Liang, B.; Li, L.; Yang, X.; Huang, Y.; Wang, A.; Wang, X.; Zhang, T. ACS Catal. 2015, 5, 1623.
doi: 10.1021/acscatal.5b00143 |
| [20] |
Kang, W.; Varma, A. Appl. Catal. B-Environ. 2018, 220, 409.
doi: 10.1016/j.apcatb.2017.08.053 |
| [21] |
Yao, Q. L.; Lu, Z. H.; Yang, K. K. Sci. Rep. 2015, 5, 15186.
doi: 10.1038/srep15186 |
| [22] |
Yao, Q.; Shi, W.; Feng, G.; Lu, Z.-H.; Zhang, X.; Tao, D.; Kong, D.; Chen, X. J. Power Sources 2014, 257, 293.
doi: 10.1016/j.jpowsour.2014.01.122 |
| [23] |
Huang, M.; Yao, Q.; Feng, G.; Zou, H.; Lu, Z. H. Inorg. Chem. 2020, 59, 5781.
doi: 10.1021/acs.inorgchem.0c00600 |
| [24] |
Pan, X. L.; Fan, Z. L.; Chen, W.; Ding, Y. J.; Luo, H. Y.; Bao, X. H. Nature Mater. 2007, 6, 507.
doi: 10.1038/nmat1916 |
| [25] |
Wang, H.; Wu, L.; Jia, A.; Li, X.; Shi, Z.; Duan, M.; Wang, Y. Chem. Eng. J. 2018, 332, 637.
doi: 10.1016/j.cej.2017.09.126 |
| [26] |
Zhang, S.; Yao, Q.; Li, Q.; Feng, G.; Lu, Z. H. Energy Technol. 2019, 7, 1800533.
doi: 10.1002/ente.v7.3 |
| [27] |
He, L.; Huang, Y.; Wang, A.; Wang, X.; Zhang, T. AICHE J. 2013, 59, 4297.
doi: 10.1002/aic.v59.11 |
| [28] |
Wang, H.; Wu, L.; Wang, Y.; Li, X.; Wang, Y. Catal. Commun. 2017, 100, 33.
doi: 10.1016/j.catcom.2017.06.021 |
| [29] |
Kim, Y.; Kwon, K. Y. Bull. Korean Chem. Soc. 2019, 40, 1167.
doi: 10.1002/bkcs.v40.12 |
| [30] |
Wang, Z. L.; Yan, J. M.; Ping, Y.; Wang, H. L.; Zheng, W. T.; Jiang, Q. Angew. Chem. Int. Ed. 2013, 52, 4406.
doi: 10.1002/anie.201301009 |
| [31] |
Lu, Z.-H.; Yao, Q.; Zhang, Z.; Yang, Y.; Chen, X. J. Nanomater. 2014, 2014, 1.
|
| [32] |
Kang, Y.; Xue, Q.; Peng, R.; Jin, P.; Zeng, J.; Jiang, J.; Chen, Y. NPG Asia Mater. 2017, 9, 407.
|
| [33] |
Liu, M.; Zheng, Y.; Xie, S.; Li, N.; Lu, N.; Wang, J.; Kim, M. J.; Guo, L.; Xia, Y. Phys. Chem. Chem. Phys. 2013, 15, 11822.
doi: 10.1039/c3cp51950c |
| [34] |
Singh, S. K.; Xu, Q. J. Am. Chem. Soc. 2009, 131, 18032.
doi: 10.1021/ja908037t |
| [35] |
Singh, A. K.; Yadav, M.; Aranishi, K.; Xu, Q. Int. J. Hydrogen Energy 2012, 37, 18915.
doi: 10.1016/j.ijhydene.2012.09.104 |
| [36] |
Zhong, D.-C.; Mao, Y.-L.; Tan, X.; Zhong, P.; Liu, L.-X. Int. J. Hydrogen Energy 2016, 41, 6362.
doi: 10.1016/j.ijhydene.2016.02.109 |
| [37] |
Zhang, Z. J.; Wang, Y. Q.; Chen, X. S.; Lu, Z. H. J. Power Sources 2015, 291, 14.
doi: 10.1016/j.jpowsour.2015.05.012 |
| [38] |
Wang, J.; Zhang, X.-B.; Wang, Z.-L.; Wang, L.-M.; Zhang, Y. Energy Environ. Sci. 2012, 5, 6885.
doi: 10.1039/c2ee03344e |
| [39] |
Hou, T.; Luo, Q.; Li, Q. Nat. Commun. 2020, 11, 4251.
doi: 10.1038/s41467-020-18091-7 |
| [40] |
Chen, S. Nanoscale 2018, 10, 20043.
doi: 10.1039/c8nr05760e pmid: 30324961 |
| [41] |
Naguib, M.; Come, J.; Dyatkin, B.; Presser, V.; Taberna, P.-L.; Simon, P.; Barsoum, M. W.; Gogotsi, Y. Electrochem. Commun. 2012, 16, 61.
doi: 10.1016/j.elecom.2012.01.002 |
| [42] |
Kang, K. M. ACS Appl. Mater. Inter. 2017, 9, 44687.
doi: 10.1021/acsami.7b10932 |
| [43] |
Kim, S. J. ACS Nano 2018, 12, 986.
doi: 10.1021/acsnano.7b07460 |
| [44] |
Cao, S.; Shen, B.; Tong, T.; Fu, J.; Yu, J. Adv. Funct. Mater 2018, 28, 1800136.
doi: 10.1002/adfm.v28.21 |
| [45] |
Liu, T.; Wang, Q.; Yuan, J.; Zhao, X.; Gao, G. ChemCatChem 2018, 10, 2200.
doi: 10.1002/cctc.v10.10 |
| [46] |
Yin, B.; Wang, Q. T.; Liu, T.; Gao, G. H. New J. Chem. 2018, 42, 20001.
doi: 10.1039/C8NJ04766A |
| [47] |
Zhang, Z.; Lu, Z.-H.; Tan, H.; Chen, X.; Yao, Q. J. Mater. Chem. A 2015, 3, 23520.
doi: 10.1039/C5TA06197K |
| [48] |
Chen, J.; Yao, Q.; Zhu, J.; Chen, X.; Lu, Z.-H. Int. J. Hydrogen Energy 2016, 41, 3946.
doi: 10.1016/j.ijhydene.2015.12.158 |
| [49] |
Singh, A. K.; Xu, Q. ChemCatChem 2013, 5, 652.
doi: 10.1002/cctc.201200591 |
| [50] |
Yao, Q.; Lu, Z. H.; Hu, Y. J.; Chen, X. S. RSC Adv. 2016, 6, 89450.
doi: 10.1039/C6RA19126F |
| [51] |
Hu, Y.; Wang, Y.; Lu, ZH.; Chen, X.; Xiong, L. Appl. Surf. Sci. 2015, 341, 185.
doi: 10.1016/j.apsusc.2015.02.094 |
| [52] |
Yoo, J. B.; Kim, H. S.; Kang, S. H.; Lee, B.; Hur, N. H. J. Mater. Chem. A 2014, 2, 18929.
doi: 10.1039/C4TA03550J |
| [53] |
Gu, X.; Lu, Z. H.; Xu, Q. Chem. Commun. 2010, 46, 7400.
doi: 10.1039/c0cc02808h |
| [54] |
Li, X. G.; Zhang, C. L.; Luo, M. H.; Yao, Q. L.; Lu, Z. H. Inorg. Chem. Front. 2020, 7, 1298.
doi: 10.1039/D0QI00073F |
| [55] |
Xia, B.; Cao, N.; Dai, H.; Su, J.; Wu, X.; Luo, W.; Cheng, G. ChemCatChem 2014, 6, 2549.
doi: 10.1002/cctc.201402353 |
| [56] |
Xia, B. Q.; Chen, K.; Luo, W.; Cheng, G. Z. Nano Res. 2015, 8, 3472.
doi: 10.1007/s12274-015-0845-4 |
| [57] |
Zhao, P.; Cao, N.; Luo, W.; Cheng, G. J. Mater. Chem. A 2015, 3, 12468.
doi: 10.1039/C5TA02201K |
| [58] |
Yang, K.; Yang, K. K.; Zhang, S. L.; Luo, Y.; Yao, Q. L.; Lu, Z. H. J. Alloys Compd. 2018, 732, 363.
doi: 10.1016/j.jallcom.2017.10.241 |
| [59] |
Zhang, Z.; Zhang, S.; Yao, Q.; Feng, G.; Zhu, M.; Lu, Z.-H. Inorg. Chem. Front. 2018, 5, 370.
doi: 10.1039/C7QI00555E |
| [60] |
Singh, S. K.; Xu, Q. Inorg. Chem. 2010, 49, 6148.
doi: 10.1021/ic1007654 |
| [61] |
Singh, S. K.; Lu, Z.-H.; Xu, Q. Eur. J. Inorg. Chem. 2011, 2011, 2232.
doi: 10.1002/ejic.201100083 |
| [62] |
Singh, A. K.; Xu, Q. Int. J. Hydrogen Energy 2014, 39, 9128.
doi: 10.1016/j.ijhydene.2014.04.001 |
| [63] |
Oliaee, S. N.; Zhang, C.; Hwang, S. Y.; Cheung, H. M.; Peng, Z. J. Phys. Chem. C 2016, 120, 9764.
doi: 10.1021/acs.jpcc.6b00815 |
| [64] |
He, L.; Huang, Y.; Wang, A.; Liu, Y.; Liu, X.; Chen, X.; Delgado, J. J.; Wang, X.; Zhang, T. J. Catal. 2013, 298, 1.
doi: 10.1016/j.jcat.2012.10.012 |
| [65] |
Jiang, Y.; Kang, Q.; Zhang, J.; Dai, H.-B.; Wang, P. J. Power Sources 2015, 273, 554.
doi: 10.1016/j.jpowsour.2014.09.119 |
| [66] |
Jiang, Y. Y.; Dai, H. B.; Zhong, Y. J.; Chen, D. M.; Wang, P. Chem. Eur. J. 2015, 21, 15439.
doi: 10.1002/chem.v21.43 |
| [67] |
Wang, H. L.; Yan, J. M.; Wang, Z. L.; O, S. I.; Jiang, Q. J. Mater. Chem. A 2013, 1, 14957.
doi: 10.1039/c3ta13259e |
| [68] |
Dai, H.; Qiu, Y.-P.; Dai, H.-B.; Wang, P. ACS Sustain. Chem. Eng. 2018, 6, 9876.
doi: 10.1021/acssuschemeng.8b01098 |
| [69] |
Zhong, Y.-J.; Dai, H.-B.; Jiang, Y.-Y.; Chen, D.-M.; Zhu, M.; Sun, L.-X.; Wang, P. J. Power Sources 2015, 300, 294.
doi: 10.1016/j.jpowsour.2015.09.071 |
| [70] |
Song, F. Z.; Zhu, Q. L.; Yang, X. C.; Zhan, W.; Pachfule, P.; Nobuko, Tsumori.; Xu, Q. Adv. Energy Mater. 2018, 8, 1701416.
doi: 10.1002/aenm.v8.1 |
| [71] |
Qiao, M. F.; Wang, Y.; Li, L. Rare Metals 2020, 39, 824.
doi: 10.1007/s12598-019-01345-9 |
| [72] |
Wang, D. C.; Lei, Y.; Jiao, W.; Liu, Y. F.; Mu, C. H.; Jian, X. Rare Metals 2021, 40, 3.
doi: 10.1007/s12598-020-01622-y |
| [73] |
Song, F. Z.; Yang, X.; Xu, Q. Small Methods 2020, 4, 1900707.
doi: 10.1002/smtd.v4.1 |
| [74] |
Song, F. Z.; Zhu, Q. L.; Nobuko, T.; Xu, Q. ACS Catal. 2015, 5, 5141.
doi: 10.1021/acscatal.5b01411 |
| [75] |
Song, F. Z.; Zhu, Q.-L.; Xu, Q. J. Mater. Chem. A 2015, 3, 23090.
doi: 10.1039/C5TA05664K |
| [76] |
Chen, J.; Lu, Z.-H.; Huang, W.; Kang, Z.; Chen, X. J. Alloy. Compd. 2017, 695, 3036.
doi: 10.1016/j.jallcom.2016.11.351 |
| [77] |
Du, Y.; Su, J.; Luo, W.; Cheng, G. ACS Appl. Mater. Inter. 2015, 7, 1031.
doi: 10.1021/am5068436 |
| [78] |
Du, X.; Du, C.; Cai, P.; Luo, W.; Cheng, G. ChemCatChem 2016, 8, 1410.
doi: 10.1002/cctc.v8.7 |
| [79] |
Singh, A.; Xu, Q. ChemCatChem 2013, 2013, 3000.
|
| [80] |
Sun, J.-K.; Xu, Q. ChemCatChem 2015, 7, 526.
doi: 10.1002/cctc.v7.3 |
| [81] |
Zhang, Z.; Zhang, S.; Yao, Q.; Chen, X.; Lu, Z. H. Inorg. Chem. 2017, 56, 11938.
doi: 10.1021/acs.inorgchem.7b01910 |
| [82] |
Zou, H. T.; Zhang, S. L.; Hong, X. L.; Yao, Q. L.; Luo, Y.; Lu, Z. H. J. Alloys Compd. 2020, 835, 155426.
doi: 10.1016/j.jallcom.2020.155426 |
| [83] |
Cao, N.; Su, J.; Luo, W.; Cheng, G. Int. J. Hydrogen Energy 2014, 39, 9726.
doi: 10.1016/j.ijhydene.2014.04.075 |
| [84] |
Cao, N.; Yang, L.; Dai, H.; Liu, T.; Su, J.; Wu, X.; Luo, W.; Cheng, G. Inorg. Chem. 2014, 53, 10122.
doi: 10.1021/ic5010352 |
| [85] |
Wen, L.; Du, X.; Su, J.; Luo, W.; Cai, P.; Cheng, G. Dalton Trans. 2015, 44, 6212.
doi: 10.1039/C5DT00493D |
| [86] |
Luo, Y.; Yang, Q.; Nie, W.; Yao, Q.; Zhang, Z.; Lu, Z. H. ACS Appl. Mater. Inter. 2020, 12, 8082.
doi: 10.1021/acsami.9b16981 |
| [87] |
Xia, B.; Liu, T.; Luo, W.; Cheng, G. J. Mater. Chem. A 2016, 4, 5616.
doi: 10.1039/C6TA00766J |
| [88] |
O, S.-I.; Yan, J.-M.; Wang, H.-L.; Wang, Z.-L.; Jiang, Q. J. Power Sources 2014, 262, 386.
doi: 10.1016/j.jpowsour.2014.03.059 |
| [89] |
Guo, F.; Zou, H.; Yao, Q.; Huang, B.; Lu, Z.-H. Renew. Energy 2020, 155, 1293.
doi: 10.1016/j.renene.2020.04.047 |
| [90] |
Singh, S. K.; Xu, Q. Chem. Commun. 2010, 46, 6545.
doi: 10.1039/c0cc01879a |
| [91] |
Qiu, Y. P.; Yin, H.; Dai, H.; Gan, L. Y.; Dai, H. B.; Wang, P. Chem. Eur. J. 2018, 24, 4902.
doi: 10.1002/chem.201705923 |
| [92] |
He, L.; Huang, Y.; Liu, X. Y.; Li, L.; Wang, A.; Wang, X.; Mou, C.-Y.; Zhang, T. Appl. Catal. B-Environ. 2014, 147, 779.
doi: 10.1016/j.apcatb.2013.10.022 |
| [93] |
Hong, X.; Yao, Q.; Huang, M.; Du, H.; Lu, Z.-H. Inorg. Chem. Front. 2019, 6, 2271.
doi: 10.1039/C9QI00848A |
| [94] |
Zhao, P.; Cao, N.; Su, J.; Luo, W.; Cheng, G. ACS Sustain. Chem. Eng. 2015, 3, 1086.
doi: 10.1021/acssuschemeng.5b00009 |
| [95] |
Singh, S. K.; Iizuka, Y.; Xu, Q. Int. J. Hydrogen Energy 2011, 36, 11794.
doi: 10.1016/j.ijhydene.2011.06.069 |
| [96] |
Bhattacharjee, D.; Mandal, K.; Dasgupta, S. J. Power Sources 2015, 287, 96.
doi: 10.1016/j.jpowsour.2015.04.008 |
| [97] |
Bhattacharjee, D.; Dasgupta, S. J. Mater. Chem. A 2015, 3, 24371.
doi: 10.1039/C5TA05814G |
| [98] |
Song-Il, O.; Yan, J.-M.; Wang, H.-L.; Wang, Z.-L.; Jiang, Q. Int. J. Hydrogen Energy 2014, 39, 3755.
doi: 10.1016/j.ijhydene.2013.12.135 |
| [99] |
Wang, K.; Yao, Q.; Qing, S.; Lu, Z.-H. J. Mater. Chem. A 2019, 7, 9903.
doi: 10.1039/c9ta01066a |
| [100] |
Firdous, N.; Janjua, N. K.; Qazi, I.; Sarwar Wattoo, M. H.. Int. J. Hydrogen Energy 2016, 41, 984.
doi: 10.1016/j.ijhydene.2015.10.084 |
| [101] |
Du, X.; Cai, P.; Luo, W.; Cheng, G. Int. J. Hydrogen Energy 2017, 42, 6137.
doi: 10.1016/j.ijhydene.2016.12.049 |
| [102] |
luo, W.; Xiao, Q.-D.; Tan, S.; Cai, P.; Cheng, G. J. Mater. Chem. A 2016, 4, 14572.
doi: 10.1039/C6TA05917A |
| [103] |
Liu, T.; Yu, J.; Bie, H.; Hao, Z. J. Alloy. Compd. 2017, 690, 783.
doi: 10.1016/j.jallcom.2016.08.113 |
| [104] |
Wang, J.; Li, W.; Wen, Y.; Gu, L.; Zhang, Y. Adv. Energy Mater. 2015, 5, 1401879.
doi: 10.1002/aenm.201401879 |
| [105] |
Yao, Q.; He, M.; Hong, X.; Chen, X.; Feng, G.; Lu, Z.-H. Int. J. Hydrogen Energy 2019, 44, 28430.
doi: 10.1016/j.ijhydene.2019.02.105 |
| [106] |
Yao, Q.; He, M.; Hong, X.; Zhang, X.; Lu, Z.-H. Inorg. Chem. Front. 2019, 6, 1546.
doi: 10.1039/C9QI00379G |
| [107] |
Yang, K. K.; Yao, Q. L.; Huang, W.; Chen, X. S.; Lu, Z. H. Int. J. Hydrogen Energy 2017, 42, 6840.
doi: 10.1016/j.ijhydene.2016.12.029 |
| [108] |
Yao, Q.; Ding, Y.; Lu, Z. H. Inorg. Chem. Front. 2020, 7, 3837.
doi: 10.1039/D0QI00766H |
| [109] |
Yao, Q.; Lu, Z.-H.; Zhang, R.; Zhang, S.; Chen, X.; Jiang, H.-L. J. Mater. Chem. A 2018, 6, 4386.
doi: 10.1039/C7TA10886A |
| [110] |
Yao, Q.; Lu, Z. H.; Huang, W.; Chen, X.; Zhu, J. J. Mater. Chem. A 2016, 4, 8579.
doi: 10.1039/C6TA02004F |
| [111] |
Singh, S. K.; Singh, A. K.; Aranishi, K.; Xu, Q. J. Am. Chem. Soc. 2011, 133, 19638.
doi: 10.1021/ja208475y |
| [112] |
Gao, W.; Li, C.; Chen, H.; Wu, M.; He, S.; Wei, M.; Evans, D. G.; Duan, X. Green Chem. 2014, 16, 1560.
doi: 10.1039/c3gc41939h |
| [113] |
Wu, D.; Wen, M.; Gu, C.; Wu, Q. ACS Appl. Mater. Inter. 2017, 9, 16103.
doi: 10.1021/acsami.7b00652 |
| [114] |
Men, Y.; Du, X.; Cheng, G.; Luo, W. Int. J. Hydrogen Energy 2017, 42, 27165.
doi: 10.1016/j.ijhydene.2017.08.214 |
| [115] |
Zou, H.; Yao, Q.; Huang, M.; Zhu, M.; Zhang, F.; Lu, Z.-H. Sustain. Energy Fuels 2019, 3, 3071.
doi: 10.1039/C9SE00547A |
| [116] |
Chen, J.; Zou, H.; Yao, Q.; Luo, M.; Li, X.; Lu, Z.-H. Appl. Surf. Sci. 2020, 501, 144247.
doi: 10.1016/j.apsusc.2019.144247 |
| [117] |
Yao, Q.; Lu, Z. H.; Zhang, Z.; Chen, X.; Lan, Y. Sci. Rep. 2014, 4, 7597.
doi: 10.1038/srep07597 |
| [118] |
Manukyan, K. V.; Cross, A.; Rouvimov, S.; Miller, J.; Mukasyan, A. S.; Wolf, E. E. Appl. Catal. A-Gen. 2014, 476, 47.
doi: 10.1016/j.apcata.2014.02.012 |
| [119] |
Wang, J.; Li, Y.; Zhang, Y. Adv. Funct. Mater. 2014, 24, 7073.
|
| [120] |
Wang, H.-L.; Yan, J.-M.; Li, S.-J.; Zhang, X.-W.; Jiang, Q. J. Mater. Chem. A 2015, 3, 121.
doi: 10.1039/C4TA05360E |
| [121] |
Yen, H.; Seo, Y.; Kaliaguine, S.; Kleitz, F. ACS Catal. 2015, 5, 5505.
doi: 10.1021/acscatal.5b00869 |
| [122] |
Kang, W.; Guo, H.; Varma, A. Appl. Catal. B-Environ. 2019, 249, 54.
doi: 10.1016/j.apcatb.2019.02.066 |
| [123] |
Ding, L.; Shu, Y.; Wang, A.; Zheng, M.; Li, L.; Wang, X.; Zhang, T. Appl. Catal. A-Gen. 2010, 385, 232.
doi: 10.1016/j.apcata.2010.07.020 |
| [124] |
Wu, D.; Wen, M.; Lin, X.; Wu, Q.; Gu, C.; Chen, H. J. Mater. Chem. A 2016, 4, 6595.
doi: 10.1039/C6TA01092J |
| [125] |
Cheng, H.; Huang, Y.; Wang, A.; Wang, X.; Zhang, T. Top. Catal. 2009, 52, 1535.
doi: 10.1007/s11244-009-9294-1 |
| [126] |
Zhang, J.; Kang, Q.; Yang, Z.; Dai, H.-B.; Zhuang, D.; Wang, P. J. Mater. Chem. A 2013, 1, 11623.
doi: 10.1039/c3ta12528a |
| [127] |
Qiu, Y.-P.; Cao, G.-X.; Wen, H.; Shi, Q.; Dai, H.; Wang, P. Int. J. Hydrogen Energy 2019, 44, 15110.
doi: 10.1016/j.ijhydene.2019.04.062 |
| [128] |
Liu, Y.; Zhang, H.; Ma, C.; Sun, N. Catalysts 2019, 9, 596.
doi: 10.3390/catal9070596 |
| [129] |
Zhao, B.; Song, J.; Ran, R.; Shao, Z. Int. J. Hydrogen Energy 2012, 37, 1133.
doi: 10.1016/j.ijhydene.2011.02.076 |
| [130] |
Song, J.; Ran, R.; Shao, Z. Int. J. Hydrogen Energy 2010, 35, 7919.
doi: 10.1016/j.ijhydene.2010.05.094 |
| [131] |
Oosawa, Y. J. Chem. Soc. Faraday Trans 1984, 80, 1507.
|
| [132] |
Abe, T.; Taira, N.; Tanno, Y.; Kikuchi, Y.; Nagai, K. Chem. Commun. 2014, 50, 1950.
doi: 10.1039/c3cc46701e |
| [133] |
Jana, M. K.; Gupta, U.; Rao, C. N. R. Dalton Trans. 2016, 45, 15137.
doi: 10.1039/C6DT02505F |
| [134] |
Zhang, P.-X.; Wang, Y.-G.; Huang, Y.-Q.; Zhang, T.; Wu, G.-S.; Li, J. Catal. Today 2011, 165, 80.
doi: 10.1016/j.cattod.2011.01.012 |
| [135] |
Block, J.; Schulz-Ekloff, G. J. Catal. 1973, 30, 327.
doi: 10.1016/0021-9517(73)90079-1 |
| [136] |
He, L.; Huang, Y. Q.; Wang, A. Q.; Wang, X. D.; Zhang, T. Chem. Ind. Eng. Prog. 2014, 33, 2956. (in Chinese)
|
|
(贺雷, 黄延强, 王爱琴, 王晓东, 张涛, 化工进展, 2014, 33, 2956.)
|
|
| [137] |
Wood, B. J.; Wise, H. J. Catal. 1975, 39, 471.
doi: 10.1016/0021-9517(75)90315-2 |
| [138] |
Falconer, J. L.; Wise, H. J. Catal. 1976, 43, 220.
doi: 10.1016/0021-9517(76)90308-0 |
| [139] |
Prasad, J.; Gland, J. L. Langmuir 1991, 7, 722.
doi: 10.1021/la00052a021 |
| [140] |
Alberas, D. J.; Kiss, J.; Liu, Z. M.; White, J. M. Surf. Sci. 1992, 278, 51.
doi: 10.1016/0039-6028(92)90583-R |
| [141] |
Ranney, J. T.; Franz, A. J.; Gland, J. L. Langmuir 1997, 13, 2731.
doi: 10.1021/la962019e |
| [142] |
Zhang, D. X.; Yin, H.; Zhong, H. F.; Gan, L. Y.; Wang, P. Int. J. Hydrogen Energy 2020, 45, 16114.
doi: 10.1016/j.ijhydene.2020.04.054 |
| [143] |
Dai, H. Ph.D. Dissertation, South China University of Technology, Guangzhou, 2019. (in Chinese)
|
|
(戴豪, 博士论文, 华南理工大学, 广州, 2019.)
|
|
| [144] |
Maurel, R.; Menezo, J. C. J. Catal. 1978, 51, 293.
doi: 10.1016/0021-9517(78)90304-4 |
| [145] |
Lu, X. Y.; Francis, S.; Motta, D.; Dimitratos, N.; Roldan, A. Phys. Chem. Chem. Phys. 2020, 22, 3883.
doi: 10.1039/C9CP06525C |
| [146] |
Deng, Z.; Lu, X.; Wen, Z.; Wei, S.; Liu, Y.; Fu, D.; Zhao, L.; Guo, W. Phys. Chem. Chem. Phys. 2013, 15, 16172.
doi: 10.1039/c3cp51948a |
| [147] |
He, Y. B.; Jia, J. F.; Wu, H. S. Appl. Surf. Sci. 2015, 327, 462.
doi: 10.1016/j.apsusc.2014.12.007 |
| [148] |
McKay, H. L.; Jenkins, S. J.; Wales, D. J. J. Phys. Chem. C 2011, 115, 17812.
doi: 10.1021/jp202155w |
| [149] |
Agusta, M. K.; David, M.; Nakanishi, H.; Kasai, H. Surf. Sci. 2010, 604, 245.
doi: 10.1016/j.susc.2009.11.012 |
| [150] |
Yin, H.; Qiu, Y. P.; Dai, H.; Gan, L. Y.; Dai, H. B.; Wang, P. J. Phys. Chem. C 2018, 122, 5443.
doi: 10.1021/acs.jpcc.7b11293 |
| [151] |
Daff, T. D.; de Leeuw, N. H. J. Mater. Chem. 2012, 22, 23210.
doi: 10.1039/c2jm34646j |
| [152] |
He, Y. B. Ph.D. Dissertation, Shanxi Normal University, Linfen, 2015. (in Chinese)
|
|
(贺艳斌, 博士论文, 山西师范大学, 临汾, 2015.)
|
|
| [153] |
Singh, S. K.; Xu, Q. Catal. Sci. Technol. 2013, 3, 1889.
doi: 10.1039/c3cy00101f |
| [154] |
Zhang, Z. J.; Lu, Z. H.; Chen, X. S. ACS Sustain. Chem. Eng. 2015, 3, 1255.
doi: 10.1021/acssuschemeng.5b00250 |
| [155] |
Chen, J. M.; Lu, Z. H.; Yao, Q. L. J. Mater. Chem. A 2018, 6, 20746.
doi: 10.1039/C8TA08050J |
| [156] |
Yao, L. H.; Li, X. G.; Peng, W. F.; Yao, Q. L.; Xia, J. H.; Lu, Z. H. Inorg. Chem. Front. 2021, 8, 1056.
doi: 10.1039/D0QI01244K |
| [157] |
Yao, Q. L.; Yang, K. K.; Nie, W. D.; Li, Y. X.; Lu, Z. H. Renewable Energy 2020, 147, 2024.
doi: 10.1016/j.renene.2019.09.144 |
| [158] |
He, L.; Liang, B.; Huang, Y.; Zhang, T. Natl. Sci. Rev. 2017, 5, 356.
doi: 10.1093/nsr/nwx123 |
| [159] |
Li, L. C.; Lan, Y. J. Ind. Catal. 1994, 1, 3. (in Chinese)
|
|
(李令成, 蓝蕴基, 工业催化, 1994, 1, 3.)
|
|
| [160] |
Zou, H. T.; Guo, F.; Luo, M. H.; Yao, Q. L.; Lu, Z. H. Int. J. Hydrogen Energy 2020, 45, 11641.
doi: 10.1016/j.ijhydene.2020.02.074 |
| [161] |
Li, S. J.; Wang, H. L.; Zhang, X. B.; Yan, J. M.; Jiang, Q. Adv. Energy. Mater 2018, 8, 1800625.
doi: 10.1002/aenm.v8.21 |
| [162] |
Zhang, S.; Yao, Q.; Lu, Z. Chem. Ind. Eng. Prog. 2017, 29, 426. (in Chinese)
|
|
(张世亮, 姚淇露, 卢章辉, 化学进展, 2017, 29, 426.)
|
|
| [163] |
Wang, W.; Hong, X.; Yao, Q.; Lu, Z.-H. J. Mater. Chem. A 2020, 8, 13694.
doi: 10.1039/D0TA05322H |
| [164] |
Guo, J. Q.; Du, Y. P.; Zhang, H. B. Acta Chim. Sinica 2020, 78, 625. (in Chinese)
doi: 10.6023/A20030053 |
|
(郭金秋, 杜亚平, 张洪波, 化学学报, 2020, 78, 625.)
doi: 10.6023/A20030053 |
|
| [165] |
Nie, W. D.; Yang, Q. F.; Lu, Z. H. J. Jiangxi Normal Univ. (Natural Sci.), 2019, 43, 416. (in Chinese)
|
|
(聂文丹, 杨齐凤, 卢章辉, 江西师范大学学报(自然科学版), 2019, 43, 416.)
|
|
| [166] |
Huang, G. J.; Chen, Z. G.; Li, M. D.; Yang, B.; Xin, M. L.; Li, S. P.; Yin, Z. J. Acta Chim. Sinica 2016, 74, 789. (in Chinese)
doi: 10.6023/A16070360 |
|
(黄国家, 陈志刚, 李茂东, 杨波, 辛明亮, 李仕平, 尹宗杰, 化学学报, 2016, 74, 789.)
doi: 10.6023/A16070360 |
|
| [167] |
Sun, J.; Liang, B. L.; Huang, Y. Q.; Wang, X. D. Catal. Today 2016, 274, 123.
doi: 10.1016/j.cattod.2016.01.031 |
| [168] |
Zhang, J. W.; Li, P.; Zhang, X. N.; Ma, X. J.; Wang, B. Acta Chim. Sinica 2020, 78, 597. (in Chinese)
doi: 10.6023/A20050153 |
|
(张晋维, 李平, 张馨凝, 马小杰, 王博, 化学学报, 2020, 78, 597.)
doi: 10.6023/A20050153 |
|
| [169] |
Fu, Q.; Yang, P.; Wang, J.; Wang, H.; Yang, L.; Zhao, X. J. Mater. Chem. A 2018, 6, 11370.
doi: 10.1039/C8TA03464H |
| [170] |
Zhao, M.; Xu, L.; Vara, M.; Elnabawy, A. O.; Gilroy, K. D.; Hood, Z. D.; Zhou, S.; Figueroa-Cosme, L.; Chi, M.; Mavrikakis, M.; Xia, Y. ACS Catal. 2018, 8, 6948.
doi: 10.1021/acscatal.8b00910 |
| [171] |
Zhang, Z.; Luo, Y.; Liu, S.; Yao, Q.; Qing, S.; Lu, Z. H. J. Mater. Chem. A 2019, 7, 21438.
doi: 10.1039/C9TA06987A |
| [172] |
Wang, W.; Lu, Z. H.; Luo, Y.; Zou, A.; Yao, Q. L.; Chen, X. S. ChemCatChem 2018, 10, 1620.
doi: 10.1002/cctc.v10.7 |
| [173] |
Nie, W. D.; Luo, Y.; Yang, Q. F.; Feng, G.; Yao, Q. L.; Lu, Z. H. Inorg. Chem. Front. 2020, 7, 709.
doi: 10.1039/C9QI01375J |
| [174] |
Yao, Q. L.; Lu, Z. H.; Wang, Y.; Chen, X.; Feng, G. J. Phys. Chem. C 2015, 119, 14167.
doi: 10.1021/acs.jpcc.5b02403 |
| [175] |
Chen, J. M.; Lu, Z. H.; Wang, Y.; Chen, X.; Zhang, L. Int. J. Hydrogen Energy 2015, 40, 4777.
doi: 10.1016/j.ijhydene.2015.02.054 |
| [176] |
Yang, Y. W.; Lu, Z. H.; Hu, Y. J.; Zhang, Z. J.; Shi, W. M.; Chen, X. RSC Adv. 2014, 4, 13749.
doi: 10.1039/C3RA47023G |
| [177] |
Yao, Q. L.; Lu, Z. H.; Jia, Y. S.; Chen, X.; Liu, X. Int. J. Hydrogen Energy 2015, 40, 2207.
doi: 10.1016/j.ijhydene.2014.12.047 |
| [178] |
Lu, Z. H.; Li, J. P.; Zhu, A. L.; Yao, Q. L.; Huang, W.; Zhou, R. Y.; Zhou, R. F.; Chen, X. Int. J. Hydrogen Energy 2013, 38, 5330.
doi: 10.1016/j.ijhydene.2013.02.076 |
| [179] |
Lu, Z. H.; Li, J. P.; Feng, G.; Yao, Q. L.; Zhang, F.; Zhou, R. Y.; Tao, D. J.; Chen, X.; Yu, Z. Q. Int. J. Hydrogen Energy 2014, 39, 13389.
doi: 10.1016/j.ijhydene.2014.04.086 |
| [180] |
Yao, Q. L.; Lu, Z. H.; Yang, Y. Nano Res. 2018, 11, 4412.
doi: 10.1007/s12274-018-2031-y |
| [181] |
Yao, Q.; Yang, K.; Hong, X.; Chen, X.; Lu, Z.-H. Catal. Sci. Technol. 2018, 8, 870.
doi: 10.1039/C7CY02365K |
| [182] |
Dong, Y. L.; Zhao, J. Q. Petrochem. Technol. 2018, 47, 883. (in Chinese)
|
|
(董永利, 赵继全, 石油化工, 2018, 47, 883.)
|
| [1] | 李雅宁, 王晓艳, 唐勇. 自由基聚合的立体选择性调控★[J]. 化学学报, 2024, 82(2): 213-225. |
| [2] | 崔国庆, 胡溢玚, 娄颖洁, 周明霞, 李宇明, 王雅君, 姜桂元, 徐春明. CO2加氢制醇类催化剂的设计制备及性能研究进展[J]. 化学学报, 2023, 81(8): 1081-1100. |
| [3] | 付信朴, 王秀玲, 王伟伟, 司锐, 贾春江. 团簇Au/CeO2的制备及其催化CO氧化反应构效关系的研究★[J]. 化学学报, 2023, 81(8): 874-883. |
| [4] | 刘建川, 李翠艳, 刘耀祖, 王钰杰, 方千荣. 高稳定二维联咔唑sp2碳共轭共价有机框架材料用于高效电催化氧还原★[J]. 化学学报, 2023, 81(8): 884-890. |
| [5] | 赵天成, 蒋鸿宇, 张琨, 徐一帆, 康欣悦, 胥鉴宸, 周旭峰, 陈培宁, 彭慧胜. 基于环烷烃/乙醇混合碳源高性能碳纳米管纤维的连续化制备[J]. 化学学报, 2023, 81(6): 565-571. |
| [6] | 王子豪, 陈敏, 陈昶乐. 不对称α-二亚胺镍催化制备聚烯烃弹性体★[J]. 化学学报, 2023, 81(6): 559-564. |
| [7] | 刘露杰, 张建, 王亮, 肖丰收. 生物质基多元醇的多相催化选择性氢解★[J]. 化学学报, 2023, 81(5): 533-547. |
| [8] | 徐斌, 韦秀芝, 孙江敏, 刘建国, 马隆龙. 原位合成氮掺杂石墨烯负载钯纳米颗粒用于催化香兰素高选择性加氢反应[J]. 化学学报, 2023, 81(3): 239-245. |
| [9] | 刘健, 欧金花, 李泽平, 蒋婧怡, 梁荣涛, 张文杰, 刘开建, 韩瑜. 金属-有机骨架衍生的Co单原子高效催化硝基芳烃氢化还原[J]. 化学学报, 2023, 81(12): 1701-1707. |
| [10] | 杨贯文, 伍广朋. 模块化双功能有机硼氮和硼磷催化体系的设计及其催化转化★[J]. 化学学报, 2023, 81(11): 1551-1565. |
| [11] | 刘金晶, 杨娜, 李莉, 魏子栋. 铂活性位空间结构调控氧还原机理的理论研究★[J]. 化学学报, 2023, 81(11): 1478-1485. |
| [12] | 李波, 周海燕, 马海燕, 黄吉玲. 亚乙基桥联双茚锆、铪配合物的合成及催化丙烯选择性齐聚研究: 茚环3-位取代基的影响[J]. 化学学报, 2023, 81(10): 1280-1294. |
| [13] | 陈治平, 孟永乐, 芦静, 周文武, 杨志远, 周安宁. Fe@Si/S-34催化剂的制备及其合成气制烯烃性能[J]. 化学学报, 2023, 81(1): 14-19. |
| [14] | 田钊炜, 达伟民, 王雷, 杨宇森, 卫敏. 第二代生物柴油制备的多相催化剂的结构设计及研究进展[J]. 化学学报, 2022, 80(9): 1322-1337. |
| [15] | 张爽, 杨成飞, 杨玉波, 冯宁宁, 杨刚. 基于废旧锂电池回收制备LixMO (x=0.79, 0.30, 0.08; M=Ni/Co/Mn)材料作为锂-氧气电池正极催化剂的电化学性能研究[J]. 化学学报, 2022, 80(9): 1269-1276. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||