化学学报 ›› 2023, Vol. 81 ›› Issue (11): 1478-1485.DOI: 10.6023/A23050221 上一篇 下一篇
所属专题: 庆祝《化学学报》创刊90周年合辑
研究论文
投稿日期:2023-05-10
发布日期:2023-08-28
作者简介:基金资助:
Jinjing Liua, Na Yangb, Li Lia( ), Zidong Weia(
), Zidong Weia( )
)
Received:2023-05-10
Published:2023-08-28
Contact:
*E-mail: About author:Supported by:文章分享

氧还原反应(ORR)的两个主要中间物种(*OH和*OOH)吸附强度不同且又存在线性比例关系, 导致对*OH和*OOH吸附强度调节的顾此失彼, 即使在最好的Pt催化剂上, ORR理论过电位约有0.45 V, *OH脱附为决速步(PDS). 本工作构建了具有三维空间结构的多低指数晶面Pt(100)-(110)-(111)模型, 多晶面交错形成的凹凸结构上, *OH和*OOH的吸附不再有线性比例关系, 达到同步优化*OH和*OOH吸附强度, 进而实现降低固有过电位的目的. 密度泛函理论计算结果表明, Pt(111)-(100)晶面交错的“凹点”因配位不饱和度最小, 对中间物种的调节最强, 吸附最弱; 同时还可平衡*OOH与*OH吸附强度, 使*O的质子化成为联合机理的PDS; 晶面交错形成的“平点-凹点”双活性位, 可催化解离机理的进行, 过电位可降低至0.27 V. 该策略有望推广到其它具有类似线性比例关系的反应中, 突破顾此失彼的症结, 实现催化活性根本性提高.
刘金晶, 杨娜, 李莉, 魏子栋. 铂活性位空间结构调控氧还原机理的理论研究★[J]. 化学学报, 2023, 81(11): 1478-1485.
Jinjing Liu, Na Yang, Li Li, Zidong Wei. Theoretical Study on the Regulation of Oxygen Reduction Mechanism by Modulating the Spatial Structure of Active Sites on Platinum★[J]. Acta Chimica Sinica, 2023, 81(11): 1478-1485.
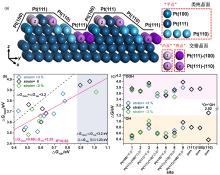



| 活性位空间结构 | 活性位晶面结构 | site | CUS[本征] | CUS[交错晶面] | CUS[per site] | ∆G*OOH/eV | ∆G*OH/eV | ||
|---|---|---|---|---|---|---|---|---|---|
| 平面 | Pt(111) | — | 3 | — | 3 | 3.74 | 0.80 | ||
| “平点” | Pt(100) | 1 | 4 | — | 4 | 3.77 | 0.51 | ||
| Pt(111) | 3 | 3 | — | 3 | 3.95 | 0.90 | |||
| Pt(110) | 6 | 5 | — | 5 | 3.53 | 0.48 | |||
| “凸点” | Pt(111)-(100) | 2 | 5 | 4 (100) | 3 (111) | 4 | 3.68 | 0.64 | |
| Pt(111)-(110) | 4 | 5 | 3 (111) | 5 (110) | 4.33 | 3.60 | 0.60 | ||
| “凹点” | Pt(111)-(110) | 8 | 3 | 5 (110) | 3 (111) | 3.67 | 3.73 | 0.61 | |
| Pt(111)-(100) | 9 | 2 | 3 (111) | 4 (100) | 3 | 3.98 | 0.97 | ||
| 活性位空间结构 | 活性位晶面结构 | site | CUS[本征] | CUS[交错晶面] | CUS[per site] | ∆G*OOH/eV | ∆G*OH/eV | ||
|---|---|---|---|---|---|---|---|---|---|
| 平面 | Pt(111) | — | 3 | — | 3 | 3.74 | 0.80 | ||
| “平点” | Pt(100) | 1 | 4 | — | 4 | 3.77 | 0.51 | ||
| Pt(111) | 3 | 3 | — | 3 | 3.95 | 0.90 | |||
| Pt(110) | 6 | 5 | — | 5 | 3.53 | 0.48 | |||
| “凸点” | Pt(111)-(100) | 2 | 5 | 4 (100) | 3 (111) | 4 | 3.68 | 0.64 | |
| Pt(111)-(110) | 4 | 5 | 3 (111) | 5 (110) | 4.33 | 3.60 | 0.60 | ||
| “凹点” | Pt(111)-(110) | 8 | 3 | 5 (110) | 3 (111) | 3.67 | 3.73 | 0.61 | |
| Pt(111)-(100) | 9 | 2 | 3 (111) | 4 (100) | 3 | 3.98 | 0.97 | ||




| 活性位结构类型 | site | η双活性位-per site/V | η单活性位/V | ∆ηa/V | ||
|---|---|---|---|---|---|---|
| “平点” | “凸点” | “凹点” | ||||
| “平点-凸点” | 12 | 0.54 | 0.72 | 0.59 | — | –0.05 |
| 23 | 0.48 | 0.64 | 0.59 | — | –0.11 | |
| 34 | 0.51 | 0.64 | 0.63 | — | –0.12 | |
| 46 | 0.71 | 0.75 | 0.63 | — | 0.08 | |
| “平点-凹点” | 68 | 0.49 | 0.75 | — | 0.62 | –0.13 |
| 91 | 0.27 | 0.72 | — | 0.46 | –0.19 |
| 活性位结构类型 | site | η双活性位-per site/V | η单活性位/V | ∆ηa/V | ||
|---|---|---|---|---|---|---|
| “平点” | “凸点” | “凹点” | ||||
| “平点-凸点” | 12 | 0.54 | 0.72 | 0.59 | — | –0.05 |
| 23 | 0.48 | 0.64 | 0.59 | — | –0.11 | |
| 34 | 0.51 | 0.64 | 0.63 | — | –0.12 | |
| 46 | 0.71 | 0.75 | 0.63 | — | 0.08 | |
| “平点-凹点” | 68 | 0.49 | 0.75 | — | 0.62 | –0.13 |
| 91 | 0.27 | 0.72 | — | 0.46 | –0.19 |
| [1] |
Fan J.; Chen M.; Zhao Z.; Zhang Z.; Ye S.; Xu S.; Wang H.; Li H. Nat. Energy 2021, 6, 475.
doi: 10.1038/s41560-021-00824-7 |
| [2] |
Jiao K.; Xuan J.; Du Q.; Bao Z.; Xie B.; Wang B.; Zhao Y.; Fan L.; Wang H.; Hou Z.; Huo S.; Brandon N. P.; Yin Y.; Guiver M. D. Nature 2021, 595, 361.
doi: 10.1038/s41586-021-03482-7 |
| [3] |
Majlan E. H.; Rohendi D.; Daud W. R. W.; Husaini T.; Haque M. A. Renewable Sustainable Energy Rev. 2018, 89, 117.
doi: 10.1016/j.rser.2018.03.007 |
| [4] |
Yan S.; Jiao L.; He C.; Jiang H. Acta Chim. Sinica 2022, 80, 1084. (in Chinese)
doi: 10.6023/A22040143 |
|
( 闫绍兵, 焦龙, 何传新, 江海龙, 化学学报, 2022, 80, 1084.)
doi: 10.6023/A22040143 |
|
| [5] |
Geng Y.; Lin X.; Sun Y.; Li H.; Qin Y.; Li C. Acta Chim. Sinica 2022, 80, 748. (in Chinese)
doi: 10.6023/A21120617 |
|
( 耿元昊, 林小秋, 孙亚昕, 李惠雨, 秦悦, 李从举, 化学学报, 2022, 80, 748.)
doi: 10.6023/A21120617 |
|
| [6] |
Wang J.; Ding W.; Wei Z. Acta Phys. Chim. Sin. 2021, 37, 2009094. (in Chinese)
|
|
( 王健, 丁炜, 魏子栋, 物理化学学报, 2021, 37, 2009094.)
|
|
| [7] |
Antoine O.; Bultel Y.; Durand R. J. Electroanal. Chem. 2001, 499, 85.
doi: 10.1016/S0022-0728(00)00492-7 |
| [8] |
Dong J.-C.; Zhang X.-G.; Briega Martos V.; Jin X.; Yang J.; Chen S.; Yang Z.-L.; Wu D.-Y.; Feliu J. M.; Williams C. T.; Tian Z.-Q.; Li J. F. Nat. Energy 2018, 4, 60.
doi: 10.1038/s41560-018-0292-z |
| [9] |
Eslamibidgoli M. J.; Huang J.; Kadyk T.; Malek A.; Eikerling M. Nano Energy 2016, 29, 334.
doi: 10.1016/j.nanoen.2016.06.004 |
| [10] |
Kulkarni A.; Siahrostami S.; Patel A.; Nørskov J. K. Chem. Rev. 2018, 118, 2302.
doi: 10.1021/acs.chemrev.7b00488 |
| [11] |
Norskov J. K.; Rossmeisl J.; Logadottir A.; Lindqvist L.; Kitchin J. R.; Bligaard T.; Jonsson H. J. Phys. Chem. B 2004, 108, 17886.
doi: 10.1021/jp047349j |
| [12] |
Bu L.; Ding J.; Guo S.; Zhang X.; Su D.; Zhu X.; Yao J.; Guo J.; Lu G.; Huang X. Adv. Mater. 2015, 27, 7204.
doi: 10.1002/adma.v27.44 |
| [13] |
Luo M.; Sun Y.; Zhang X.; Qin Y.; Li M.; Li Y.; Li C.; Yang Y.; Wang L.; Gao P.; Lu G.; Guo S. Adv. Mater. 2018, 30, 1705515.
doi: 10.1002/adma.v30.10 |
| [14] |
Wang X. X.; Sokolowski J.; Liu H.; Wu G. Chin. J. Catal. 2020, 41, 739.
doi: 10.1016/S1872-2067(19)63407-8 |
| [15] |
Yao Z.; Yuan Y.; Cheng T.; Gao L.; Sun T.; Lu Y.; Zhou Y. G.; Galindo P. L.; Yang Z.; Xu L.; Yang H.; Huang H. Nano Lett. 2021, 21, 9354.
doi: 10.1021/acs.nanolett.1c03805 |
| [16] |
Hu Y.; Guo X.; Shen T.; Zhu Y.; Wang D. ACS Catal. 2022, 12, 5380.
doi: 10.1021/acscatal.2c01541 |
| [17] |
Li Z.; Li B.; Hu Y.; Wang S.; Yu C. Mater. Adv. 2022, 3, 779.
doi: 10.1039/D1MA00858G |
| [18] |
Stamenkovic V.; Mun B. S.; Mayrhofer K. J.; Ross P. N.; Markovic N. M.; Rossmeisl J.; Greeley J.; Norskov J. K. Angew. Chem., Int. Ed. 2006, 45, 2897.
doi: 10.1002/anie.v45:18 |
| [19] |
Stamenkovic V. R.; Mun B. S.; Arenz M.; Mayrhofer K. J.; Lucas C. A.; Wang G.; Ross P. N.; Markovic N. M. Nat. Mater. 2007, 6, 241.
doi: 10.1038/nmat1840 pmid: 17310139 |
| [20] |
Wang S.; Xu W.; Zhu Y.; Luo Q.; Zhang C.; Tang S.; Du Y. ACS Appl. Mater. Interfaces 2020, 13, 827.
doi: 10.1021/acsami.0c21348 |
| [21] |
Wang Z.; Yao X.; Kang Y.; Miao L.; Xia D.; Gan L. Adv. Funct. Mater. 2019, 29, 19029.
|
| [22] |
Zeng W.-J.; Wang C.; Yan Q.-Q.; Yin P.; Tong L.; Liang H.-W. Nat. Commun. 2022, 13, 7654.
doi: 10.1038/s41467-022-35457-1 |
| [23] |
Li J.; Feng X.; Wei Z. J. Electrochem. 2018, 24, 589. (in Chinese)
|
|
( 李静, 冯欣, 魏子栋, 电化学, 2018, 24, 589.)
doi: 10.13208/j.electrochem.180850 |
|
| [24] |
He T.; Wang W.; Shi F.; Yang X.; Li X.; Wu J.; Yin Y.; Jin M. Nature 2021, 598, 76.
doi: 10.1038/s41586-021-03870-z |
| [25] |
Gamboaaldeco M. E.; Herrero E.; Zelenay P. S.; Wieckowski A. J. Electroanal. Chem. 1993, 348, 451.
doi: 10.1016/0022-0728(93)80151-7 |
| [26] |
Markovic N. M.; Adzic R. R.; Cahan B. D.; Yeager E. B. J. Electroanal. Chem. 1994, 377, 249.
doi: 10.1016/0022-0728(94)03467-2 |
| [27] |
Seh Z. W.; Kibsgaard J.; Dickens C. F.; Chorkendorff I.; Nørskov J. K.; Jaramillo T. F. Science 2017, 355, 146.
|
| [28] |
Wang C.; Daimon H.; Lee Y.; Kim J.; Sun S. J. Am. Chem. Soc. 2007, 129, 6974.
doi: 10.1021/ja070440r |
| [29] |
Yu T.; Kim D. Y.; Zhang H.; Xia Y. Angew. Chem., Int. Ed. 2011, 50, 2773.
doi: 10.1002/anie.v50.12 |
| [30] |
Corona B.; Howard M.; Zhang L.; Henkelman G. J. Chem. Phys. 2016, 145, 244708.
doi: 10.1063/1.4972579 |
| [31] |
Dang D.; Zhang L.; Zeng X.-Y.; Tian X.-L.; Qu C.; Nan H. X.; Shu T.; Hou S. Y.; Yang L. J.; Zeng J. H.; Liao S. J. J. Power Sources 2017, 355, 83.
doi: 10.1016/j.jpowsour.2017.04.050 |
| [32] |
Pan Y. T.; Li D. G.; Sharma S.; Wang C. Y.; Zachman M. J.; Wegener E. C.; Kropf A. J.; Kim Y. S.; Myers D. J.; Peterson A. A.; Cullen D. A.; Spendelow J. S. Chem. Catal. 2022, 2, 3559.
|
| [33] |
Wang D.; Xin H. L.; Hovden R.; Wang H.; Yu Y.; Muller D. A.; DiSalvo F. J.; Abruna H. D. Nat. Mater. 2013, 12, 81.
doi: 10.1038/nmat3458 |
| [34] |
Zhang G.; Shao Z. G.; Lu W.; Li G.; Liu F.; Yi B. Electrochem. Commun. 2012, 22, 145.
doi: 10.1016/j.elecom.2012.05.030 |
| [35] |
Chang Q.-W.; Xiao F.; Xu Y.; Shao M.-H. Acta Phys. -Chim. Sin. 2017, 33, 9. (in Chinese)
doi: 10.3866/PKU.WHXB201609202 |
|
( 常乔婉, 肖菲, 徐源, 邵敏华, 物理化学学报, 2017, 33, 9.)
|
|
| [36] |
Zhu H.; Luo M.-C.; Cai Y.-Z.; Sun Z.-N. Acta Phys. -Chim. Sin. 2016, 32, 2462. (in Chinese)
doi: 10.3866/PKU.WHXB201606293 |
|
( 朱红, 骆明川, 蔡业政, 孙照楠, 物理化学学报, 2016, 32, 2462.)
|
|
| [37] |
Liu J.; Liu H.; Chen H.; Du X.; Wang W. Adv. Sci. 2019, 7, 1901614.
doi: 10.1002/advs.v7.1 |
| [38] |
Sours T.; Patel A.; Norskov J.; Siahrostami S.; Kulkarni A. J. Phys. Chem. Lett. 2020, 11, 10029.
doi: 10.1021/acs.jpclett.0c02889 |
| [39] |
Lee D. G.; Kim S. H.; Lee H. H.; Shin S.; Lee J.; Joo S. H.; Lee Y.; Kwak S. K.; Song H. K. ACS Catal. 2021, 11, 12712.
doi: 10.1021/acscatal.1c02934 |
| [40] |
Wan H.; Østergaard T. M.; Arnarson L.; Rossmeisl J. ACS Sustainable Chem. Eng. 2018, 7, 611.
doi: 10.1021/acssuschemeng.8b04173 |
| [41] |
Zou W.; Lu R.; Liu X.; Xiao G.; Liao X.; Wang Z.; Zhao Y. J. Mater. Chem. A 2022, 10, 9150.
doi: 10.1039/D2TA00313A |
| [42] |
Zheng X.; Li L.; Li J.; Wei Z. Phys. Chem. Chem. Phys. 2019, 21, 3242.
doi: 10.1039/C8CP07556E |
| [43] |
Zhang Q.; Asthagiri A. Catal. Today 2019, 323, 35.
doi: 10.1016/j.cattod.2018.07.036 |
| [44] |
Seh Z. W.; Kibsgaard J.; Dickens C. F.; Chorkendorff I. B.; Norskov J. K.; Jaramillo T. F. Science 2017, 355, 1.
|
| [45] |
Xu H.-X.; Cheng D.-J.; Cao D.-P.; Zeng X.-C. Nat. Catal. 2018, 1, 339.
doi: 10.1038/s41929-018-0063-z |
| [46] |
Zhou Y.-Z.; Lu R.-H.; Tao X.-F.; Qiu Z.-J.; Chen G.-B.; Yang J.; Zhao Y.; Feng X.-L.; Müllen K. J. Am. Chem. Soc. 2023, 145, 3647.
doi: 10.1021/jacs.2c12933 |
| [1] | 黄广龙, 薛小松. “陈试剂”作为三氟甲基源机理的理论研究[J]. 化学学报, 2024, 82(2): 132-137. |
| [2] | 李萍, 杨琪玉, 曾婧, 张然, 陈秋燕, 闫飞. 氟掺杂对可逆固体氧化物电池性能的影响及相关动力学研究[J]. 化学学报, 2024, 82(1): 36-45. |
| [3] | 梁雪峰, 荆剑, 冯昕, 赵勇泽, 唐新员, 何燕, 张立胜, 李慧芳. 共价有机框架COF66/COF366的电子结构: 从单体到二维平面聚合物[J]. 化学学报, 2023, 81(7): 717-724. |
| [4] | 杨磊, 葛娇阳, 王访丽, 吴汪洋, 郑宗祥, 曹洪涛, 王洲, 冉雪芹, 解令海. 一种基于芴的大环结构的有效降低内重组能的理论研究[J]. 化学学报, 2023, 81(6): 613-619. |
| [5] | 张少秦, 李美清, 周中军, 曲泽星. 多共振热激活延迟荧光过程的理论研究[J]. 化学学报, 2023, 81(2): 124-130. |
| [6] | 王娟, 肖华敏, 谢丁, 郭元茹, 潘清江. 铜掺杂与氮化碳复合氧化锌材料结构和二氧化氮气体传感性质的密度泛函理论计算[J]. 化学学报, 2023, 81(11): 1493-1499. |
| [7] | 闫绍兵, 焦龙, 何传新, 江海龙. ZIF-67/石墨烯复合物衍生的氮掺杂碳限域Co纳米颗粒用于高效电催化氧还原[J]. 化学学报, 2022, 80(8): 1084-1090. |
| [8] | 王丹, 封波, 张晓昕, 刘亚楠, 裴燕, 乔明华, 宗保宁. 基于热解ZIF-8的氮掺杂碳电化学氧还原合成过氧化氢催化剂[J]. 化学学报, 2022, 80(6): 772-780. |
| [9] | 耿元昊, 林小秋, 孙亚昕, 李惠雨, 秦悦, 李从举. 双金属导电金属有机框架材料Ni/Co-CAT的制备及其氧还原催化性能研究[J]. 化学学报, 2022, 80(6): 748-755. |
| [10] | 栾雪菲, 王聪芝, 夏良树, 石伟群. 铀酰与羧酸和肟基类配体相互作用的理论研究[J]. 化学学报, 2022, 80(6): 708-713. |
| [11] | 王珞聪, 李哲伟, 岳彩巍, 张培焕, 雷鸣, 蒲敏. 电场下偶氮苯衍生物分子顺反异构化反应机理的理论研究[J]. 化学学报, 2022, 80(6): 781-787. |
| [12] | 于潇涵, 黄伟, 李彦光. 二维共价有机框架材料的可控合成及其光催化应用研究进展[J]. 化学学报, 2022, 80(11): 1494-1506. |
| [13] | 王英辉, 魏思敏, 段金伟, 王康. 理论研究“受阻路易斯酸碱对”催化的烯醇硅醚氢化反应机理[J]. 化学学报, 2021, 79(9): 1164-1172. |
| [14] | 熊昆, 陈伽瑶, 杨娜, 蒋尚坤, 李莉, 魏子栋. 理论探究水溶液条件对TMNxCy催化氮还原性能的增强机制[J]. 化学学报, 2021, 79(9): 1138-1145. |
| [15] | 满清敏, 付尊蕴, 刘甜甜, 郑明月, 蒋华良. Cu催化偶联反应合成烷基芳基醚的DFT机理研究[J]. 化学学报, 2021, 79(7): 948-952. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||