化学学报 ›› 2021, Vol. 79 ›› Issue (11): 1303-1319.DOI: 10.6023/A21070345 上一篇 下一篇
综述
投稿日期:2021-07-26
发布日期:2021-09-17
通讯作者:
肖检, 彭羽
作者简介: |
尚阳, 1995年出生于四川南充, 2018年在重庆邮电大学获得学士学位. 2018年至2021年, 在彭羽教授指导下攻读并获得硕士学位. 研究兴趣是木脂素天然产物的全合成. |
 |
肖检, 1990年出生于四川巴中, 西南交通大学生命科学与工程学院助理研究员. 2018年在兰州大学功能有机分子化学国家重点实验室获得博士学位, 导师为彭羽教授. 2018年加入西南交通大学生命科学与工程学院化学系, 主要研究方向包括活性天然产物和药物分子的全合成、有机合成新方法发展等. |
 |
王雅雯, 西南交通大学生命科学与工程学院教授. 2000、2005年在兰州大学分别获得学士和博士学位, 导师为刘伟生教授. 曾任兰州大学化学化工学院教授. 主要研究领域为小分子荧光探针的合成及生物成像、超分子化学等. |
 |
彭羽, 西南交通大学生命科学与工程学院教授. 2000、2005年在兰州大学分别获得学士和博士学位, 导师为李卫东教授. 2008年至2009年在美国内华达大学里诺分校和加州大学圣芭芭拉分校做博士后研究, 合作导师为Liming Zhang教授. 曾任兰州大学化学化工学院和功能有机分子化学国家重点实验室教授. 主要研究领域为活性天然产物和药物分子的全合成、镍催化的还原环化新方法发展等. |
基金资助:
Yang Shang, Jian Xiao( ), Yawen Wang, Yu Peng(
), Yawen Wang, Yu Peng( )
)
Received:2021-07-26
Published:2021-09-17
Contact:
Jian Xiao, Yu Peng
Supported by:文章分享
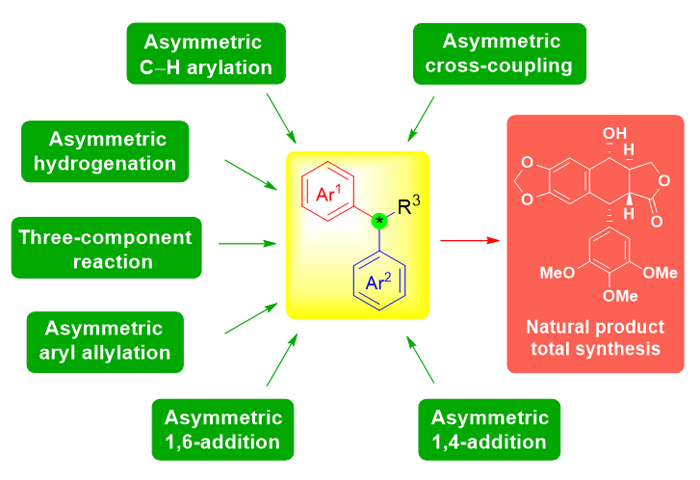
二芳基次甲基结构单元广泛存在于具有重要生理和药理活性的天然产物和药物当中. 同时, 该结构单元中所特有的二芳基次甲基立体中心的对映选择性构筑往往也是天然产物全合成的难点和挑战性所在. 因此, 引起了众多有机合成化学家的研究兴趣. 近年来, 该手性立体中心的构建方法发展迅速, 新方法和新反应的报道也层出不穷; 开发出来的一些高效催化剂, 展示出独特的催化活性和选择性. 本文根据反应类型的不同, 将其分为不对称共轭加成反应和不对称氢化反应等六类, 综述近十年来二芳基次甲基立体中心的不对称构建方法及相关方法在天然产物全合成中的应用. 最后, 从全合成的角度进一步总结和分析未来构建二芳基次甲基手性立体中心的发展趋势, 力求发展更加高效、避免贵金属的催化剂及环境友好型的新方法和新试剂.
尚阳, 肖检, 王雅雯, 彭羽. 不对称构筑二芳基次甲基立体中心的研究进展[J]. 化学学报, 2021, 79(11): 1303-1319.
Yang Shang, Jian Xiao, Yawen Wang, Yu Peng. Advances on Asymmetric Construction of Diarylmethine Stereocenters[J]. Acta Chimica Sinica, 2021, 79(11): 1303-1319.
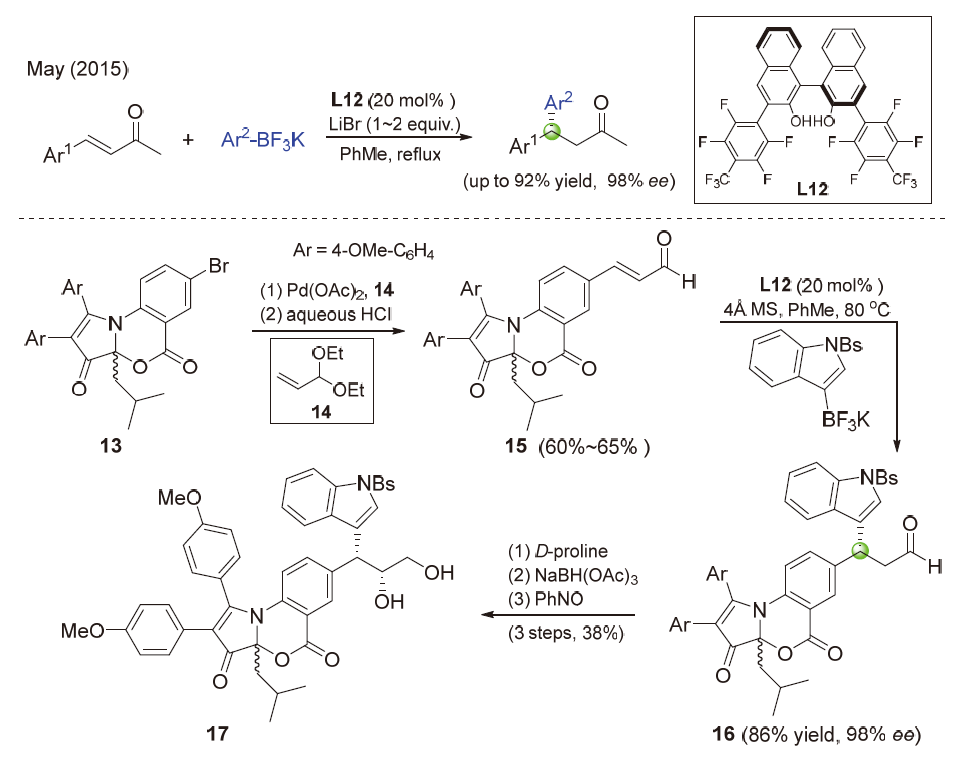
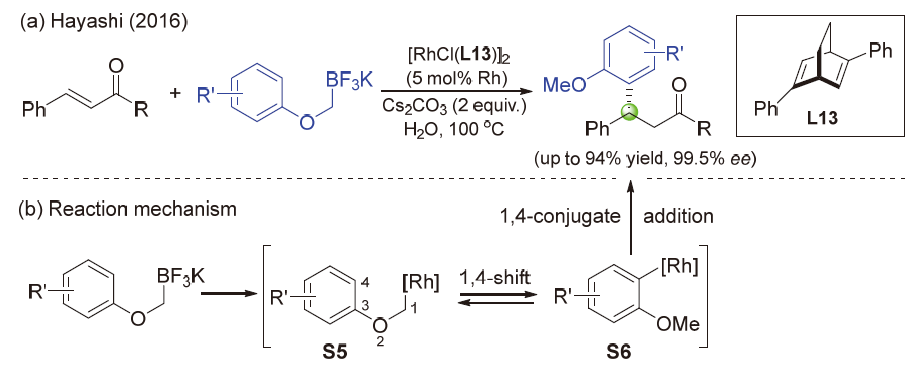
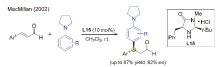
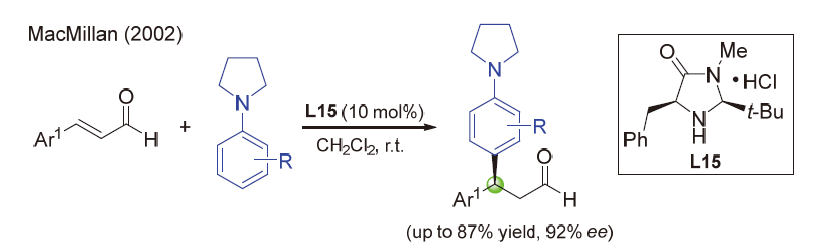
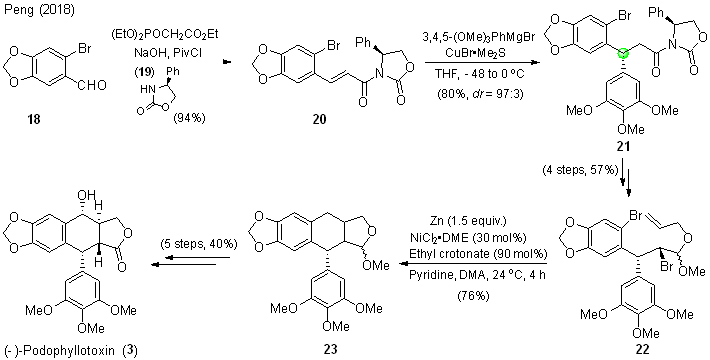
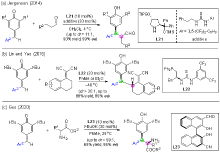
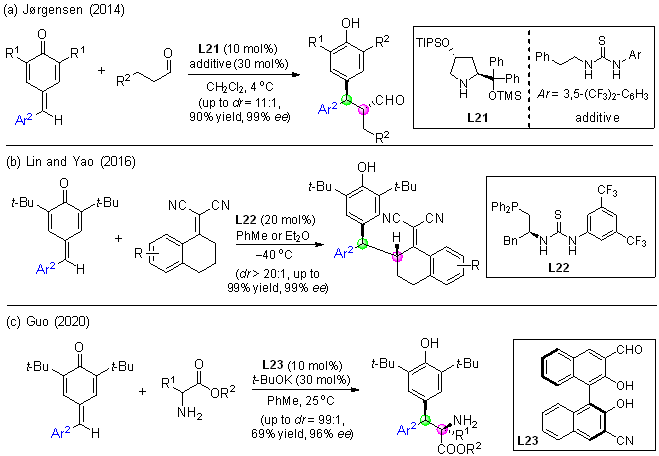
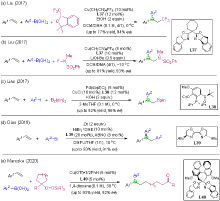
| [1] |
Lindsay De Vane C.; Liston H. L.; Markowitz J. S. Clin Pharmacokinet. 2002, 41, 1247.
pmid: 12452737 |
| [2] |
Hyttel J.; Larsen J. J. J. Neurochem. 1985, 44, 1615.
pmid: 2580950 |
| [3] |
(a) Stähelin H. F.; von Wartburg A. Cancer Res. 1991, 51, 5.
pmid: 1988106 |
|
(b) Liu Y.-Q.; Yang L.; Tian X. Curr. Bioact. Compd. 2007, 3, 37.
doi: 10.2174/157340707780126499 pmid: 1988106 |
|
| [4] |
Sakai M.; Hayashi H.; Miyaura N. Organometallics 1997, 16, 4229.
doi: 10.1021/om9705113 |
| [5] |
Hayashi T.; Tokunaga N.; Okamoto K.; Shintani R. Chem. Lett. 2005, 34, 1480.
doi: 10.1246/cl.2005.1480 |
| [6] |
Paquin J.-F.; Defieber C.; Stephenson C. R. J.; Carreira E. M. J. Am. Chem. Soc. 2005, 127, 10850.
doi: 10.1021/ja053270w |
| [7] |
Yao J.; Yin L.; Shen Y.; Lu T.; Hayashi T.; Dou X. Org. Lett. 2018, 20, 6882.
doi: 10.1021/acs.orglett.8b03021 |
| [8] |
Wang Z.-Q.; Feng C.-G.; Zhang S.-S.; Xu M.-H.; Lin G.-Q. Angew. Chem. Int. Ed. 2010, 49, 5780.
doi: 10.1002/anie.v49:33 |
| [9] |
Lang F.; Chen G.; Li L.; Xing J.; Han F.; Cun L.; Liao J. Chem. Eur. J. 2011, 17, 5242.
doi: 10.1002/chem.v17.19 |
| [10] |
Jumde V. R.; Iuliano A. Adv. Synth. Catal. 2013, 355, 3475.
doi: 10.1002/adsc.201300619 |
| [11] |
He Q.; Xie F.; Fu G.; Quan M.; Shen C.; Yang G.; Gridnev I. D.; Zhang W. Org. Lett. 2015, 17, 2250.
doi: 10.1021/acs.orglett.5b00863 |
| [12] |
Bao X.; Cao Y.-X.; Chu W.-D.; Qu H.; Du J.-Y.; Zhao X.-H.; Ma X.-Y.; Wang C.-T.; Fan C.-A. Angew. Chem. Int. Ed. 2013, 52, 14167.
doi: 10.1002/anie.201307324 |
| [13] |
(a) Wang J.; Wang M.; Cao P.; Jiang L.; Chen G.; Liao J. Angew. Chem. Int. Ed. 2014, 53, 6673.
doi: 10.1002/anie.201403325 |
|
(b) Han Z.; Wang Z.; Zhang X.; Ding K. Angew. Chem. Int. Ed. 2009, 48, 5345.
doi: 10.1002/anie.200901630 |
|
| [14] |
Lee A.; Kim H. J. Am. Chem. Soc. 2015, 137, 11250.
doi: 10.1021/jacs.5b07034 |
| [15] |
(a) Takatsu K.; Shintani R.; Hayashi T. Angew. Chem. Int. Ed. 2011, 50, 5548.
doi: 10.1002/anie.201008196 |
|
(b) Davies H. M.; Gregg T. M. Tetrahedron Lett. 2002, 43, 4951.
doi: 10.1016/S0040-4039(02)00941-3 |
|
| [16] |
Shih J.-L.; Nguyen T. S.; May J. A. Angew. Chem. Int. Ed. 2015, 54, 9931.
doi: 10.1002/anie.v54.34 |
| [17] |
Ming J.; Hayashi T. Org. Lett. 2016, 18, 6452.
doi: 10.1021/acs.orglett.6b03347 |
| [18] |
Wu C.; Yue G.; Nielsen C. D.-T.; Xu K.; Hirao H.; Zhou J. J. Am. Chem. Soc. 2016, 138, 742.
doi: 10.1021/jacs.5b11441 |
| [19] |
Paras N. A.; MacMillan D. W. C. J. Am. Chem. Soc. 2002, 124, 7894.
pmid: 12095321 |
| [20] |
Hong L.; Wang L.; Sun W.; Wong K.; Wang R. J. Org. Chem. 2009, 74, 6881.
doi: 10.1021/jo901409d |
| [21] |
Zhang H.; Liao Y.-H.; Yuan W.-C.; Zhang X.-M. Eur. J. Org. Chem. 2010, 3215.
|
| [22] |
Evans D. A.; Barotroli J.; Shih T. L. J. Am. Chem. Soc. 1981, 103, 2127.
doi: 10.1021/ja00398a058 |
| [23] |
Xiao J.; Cong X.-W.; Yang G.-Z.; Wang Y.-W.; Peng Y. Org. Lett. 2018, 20, 1651.
doi: 10.1021/acs.orglett.8b00408 |
| [24] |
(a) Peng Y.; Luo Z.-B.; Zhang J.-J.; Luo L.; Wang Y.-W. Org. Biomol. Chem. 2013, 11, 7574.
doi: 10.1039/c3ob41672k pmid: 27658859 |
|
(b) Zhang J.-J.; Yan C.-S.; Peng Y.; Luo Z.-B.; Xu X.-B.; Wang Y.-W. Org. Biomol. Chem. 2013, 11, 2498.
doi: 10.1039/c3ob00053b pmid: 27658859 |
|
|
(c) Peng Y.; Xiao J.; Xu X.-B.; Duan S.-M.; Ren L.; Shao Y.-L.; Wang Y.-W. Org. Lett. 2016, 18, 5170.
pmid: 27658859 |
|
|
(d) Luo Z.-B.; Wang Y.-W.; Peng Y. Org. Biomol. Chem. 2020, 18, 2054.
doi: 10.1039/D0OB00376J pmid: 27658859 |
|
| [25] |
(a) Chu W.-D.; Zhang L.-F.; Bao X.; Zhao X.-H; Zeng C.; Du J.-Y.; Zhang G.-B.; Wang F.-X.; Ma X.-Y.; Fan C.-A. Angew. Chem. Int. Ed. 2013, 52, 9229.
doi: 10.1002/anie.201303928 |
|
(b) Lou Y.; Cao P.; Jia T.; Zhang Y.; Wang M.; Liao J. Angew. Chem. Int. Ed. 2015, 54, 12134.
doi: 10.1002/anie.201505926 |
|
|
(c) Li S.; Liu Y.; Huang B.; Zhou T.; Tao H.; Xiao Y.; Liu L.; Zhang J. ACS Catal. 2017, 7, 2805.
doi: 10.1021/acscatal.7b00030 |
|
|
(d) Caruana L.; Kniep F.; Johansen T. K.; Poulsen P. H.; Jørgensen K. A. J. Am. Chem. Soc. 2014, 136, 15929.
doi: 10.1021/ja510475n |
|
|
(e) Li X.; Xu X.; Wei W.; Lin A.; Yao H. Org. Lett. 2016, 18, 428.
doi: 10.1021/acs.orglett.5b03471 |
|
|
(f) Wen W.; Luo M.-J.; Yuan Y.; Liu J.-H.; Wu Z.-L.; Cai T.; Wu Z.-W.; Ouyang Q.; Guo Q.-X. Nat. Commun. 2020, 11, 5372.
doi: 10.1038/s41467-020-19245-3 |
|
| [26] |
(a) Falciola C. A.; Alexakis A. Angew. Chem. Int. Ed. 2007, 46, 2619;
doi: 10.1002/(ISSN)1521-3773 |
|
(b) Kacprzynski M. A.; May T. L.; Kazane S. A. Hoveyda A. H. Angew. Chem. Int. Ed. 2007, 46, 4554.
doi: 10.1002/(ISSN)1521-3773 |
|
| [27] |
Shintani R.; Takatsu K.; Takeda M.; Hayashi T. Angew. Chem. Int. Ed. 2011, 50, 8656.
doi: 10.1002/anie.201103581 |
| [28] |
Tian H.; Zhang P.; Peng F.; Yang H.; Fu H. Org. Lett. 2017, 19, 3775.
doi: 10.1021/acs.orglett.7b01631 pmid: 28661154 |
| [29] |
Shao L.; Hu X.-P. Org. Biomol. Chem. 2017, 15, 9837.
doi: 10.1039/C7OB02133J |
| [30] |
(a) Cheng R.; Sang X.; Gao X.; Zhang S.; Xue X.; Zhang X. Angew. Chem. Int. Ed. 2021, 60, 12386.
doi: 10.1002/anie.v60.22 |
|
(b) Li X.; Gao X.; He C.; Zhang X. Org. Lett. 2021, 23, 1400.
doi: 10.1021/acs.orglett.1c00058 |
|
|
(c) Weix D. J. Acc. Chem. Res. 2015, 48, 1767.
doi: 10.1021/acs.accounts.5b00057 |
|
|
(d) Ackerman L. K. G.; Lovell M. M.; Weix D. J. Nature 2015, 524, 454.
doi: 10.1038/nature14676 |
|
|
(e) León T.; Correa A.; Martin R. Nature 2017, 545, 84.
doi: 10.1038/nature22316 |
|
|
(f) Li Z.-Q.; Wu D.; Ding C.; Yin G.-Y. CCS Chem. 2020, 2, 576.
|
|
|
(g) Belal M.; Li Z.-Q.; Lu X.-Q.; Yin G.-Y. Sci. China Chem. 2021, 64, 513.
doi: 10.1007/s11426-020-9910-2 |
|
| [31] |
(a) Sun D.; Ma G.; Zhao X.; Lei C.; Gong H. Chem. Sci. 2021, 12, 5253.
doi: 10.1039/D1SC00283J |
|
(b) Pan Q.; Ping Y.; Wang Y.; Guo Y.; Kong W. J. Am. Chem. Soc. 2021, 143, 10282.
doi: 10.1021/jacs.1c03827 |
|
|
(c) DeLano T. J.; Dibrell S. E.; Lacker C. R.; Pancoast A. R.; Poremba K. E.; Cleary L.; Sigman M. S.; Reisman S. E. Chem. Sci. 2021, 12, 7758.
doi: 10.1039/D1SC00822F |
|
|
(d) Fan P.; Lan Y.; Zhang C.; Wang C. J. Am. Chem. Soc. 2020, 142, 2180.
doi: 10.1021/jacs.9b12554 |
|
|
(e) Wang Z.; Yang Z.-P.; Fu G. C. Nat. Chem. 2021, 13, 236.
doi: 10.1038/s41557-020-00609-7 |
|
| [32] |
(a) Do H.-Q.; Chandrashekar E. R. R.; Fu G. C. J. Am. Chem. Soc. 2013, 135, 16288.
doi: 10.1021/ja408561b pmid: 23039358 |
|
(b) Wilsily A.; Tramutola F.; Owston N. A.; Fu G. C. J. Am. Chem. Soc. 2012, 134, 5794.
doi: 10.1021/ja301612y pmid: 23039358 |
|
|
(c) Binder J. T.; Cordier C. J.; Fu G. C. J. Am. Chem. Soc. 2012, 134, 17003.
doi: 10.1021/ja308460z pmid: 23039358 |
|
| [33] |
Woods B. P.; Orlandi M.; Huang C.-Y.; Sigman M. S.; Doyle A. G. J. Am. Chem. Soc. 2017, 139, 5688.
doi: 10.1021/jacs.7b03448 |
| [34] |
(a) Poremba K. E.; Kadunce N. T.; Suzuki N.; Cherney A. H.; Reisman S. E. J. Am. Chem. Soc. 2017, 139, 5684.
doi: 10.1021/jacs.7b01705 pmid: 23634932 |
|
(b) Cherney A. H.; Kadunce N. T.; Reisman S. E. J. Am. Chem. Soc. 2013, 135, 7442.
doi: 10.1021/ja402922w pmid: 23634932 |
|
| [35] |
(a) Li B.; Aliyu M. A.; Gao Z.; Li T.; Dong W.; Li J.; Shi E.; Tang W. Org. Lett. 2020, 22, 4974.
doi: 10.1021/acs.orglett.0c01489 |
|
(b) Huang K.-C.; Gopula B.; Kuo T.-S.; Chiang C.-W.; Wu P.-Y.; Henschke J. P.; Wu H.-L. Org. Lett. 2013, 15, 5730.
doi: 10.1021/ol4027599 |
|
| [36] |
(a) Yue G.; Lei K.; Hirao H.; Zhou J. Angew. Chem. Int. Ed. 2015, 54, 6531.
doi: 10.1002/anie.201501712 |
|
(b) Qin X.; Lee M. W. Y.; Zhou J. Org. Lett. 2019, 21, 5990. For a review, see:
doi: 10.1021/acs.orglett.9b02130 |
|
|
(c) Oxtoby L. J.; Gurak J. A. Jr.; Wisniewski S. R.; Eastgate M. D.; Engle K. M. Trends Chem. 2019, 1, 572.
doi: 10.1016/j.trechm.2019.05.007 |
|
| [37] |
Chen G.; Gong W.; Zhuang Z.; Andrä M. S.; Chen Y.-Q.; Hong X.; Yang Y.-F.; Liu T.; Houk K. N.; Yu J. Q. Science 2016, 353, 1023.
doi: 10.1126/science.aaf4434 |
| [38] |
Zhang W.; Wu L.; Chen P.; Liu G. Angew. Chem. Int. Ed. 2019, 58, 6425.
doi: 10.1002/anie.v58.19 |
| [39] |
Cheng X.; Lu H.; Lu Z. Nature Commun. 2019, 10, 3549.
doi: 10.1038/s41467-019-11392-6 |
| [40] |
Yamamoto E.; Hilton M. J.; Orlandi M.; Saini V.; Toste F. D.; Sigman M. S. J. Am. Chem. Soc. 2016, 138, 15877.
pmid: 27960315 |
| [41] |
(a) Wu L.; Wang F.; Wan X.; Wang D.; Chen P.; Liu G. J. Am. Chem. Soc. 2017, 139, 2904.
doi: 10.1021/jacs.6b13299 |
|
(b) Wang D.; Wu L.; Wang F.; Wan X.; Chen P.; Lin Z.; Liu G. J. Am. Chem. Soc. 2017, 139, 6811.
doi: 10.1021/jacs.7b02455 |
|
| [42] |
Chen B.; Cao P.; Yin X.; Liao Y.; Jiang L.; Ye J.; Wang M.; Liao J. ACS Catal. 2017, 7, 2425.
doi: 10.1021/acscatal.7b00300 |
| [43] |
Anthony D.; Lin Q.; Baudet J.; Diao T. Angew. Chem. Int. Ed. 2019, 58, 3198.
doi: 10.1002/anie.v58.10 |
| [44] |
Sakurai S.; Matsumoto A.; Kano T.; Maruoka K. J. Am. Chem. Soc. 2020, 142, 19017.
doi: 10.1021/jacs.0c09008 |
| [45] |
Song S.; Zhu S.-F.; Yu Y.-B.; Zhou Q.-L. Angew. Chem. Int. Ed. 2013, 52, 1556.
doi: 10.1002/anie.201208606 |
| [46] |
Li Y.; Dong K.; Wang Z.; Ding K. Angew. Chem. Int. Ed. 2013, 52, 6748.
doi: 10.1002/anie.v52.26 |
| [47] |
Nie H.; Zhu Y.; Hu X.; Wei Z.; Yao L.; Zhou G.; Wang P.; Jiang R.; Zhang S. Org. Lett. 2019, 21, 8641.
doi: 10.1021/acs.orglett.9b03251 |
| [48] |
Xu B.; Li M.-L.; Zuo X.-D.; Zhu S.-F.; Zhou Q.-L. J. Am. Chem. Soc. 2015, 137, 8700.
doi: 10.1021/jacs.5b05086 |
| [49] |
Zhu D.-X.; Xia H.; Liu J.-G.; Chung L.-W.; Xu M.-H. J. Am. Chem. Soc. 2021, 143, 2608.
doi: 10.1021/jacs.0c13191 |
| [50] |
Guo Q. Chin. J. Org. Chem. 2019, 39, 2912. (in Chinese)
doi: 10.6023/cjoc201902026 |
|
( 郭庆君, 有机化学 2019, 39, 2912.)
doi: 10.6023/cjoc201902026 |
| [1] | 张大伟, 赵海洋, 冯笑甜, 顾玉诚, 张新刚. 钯催化下杂芳基溴代物与偕二氟烯丙基硼试剂的交叉偶联反应[J]. 化学学报, 2024, 82(2): 105-109. |
| [2] | 杨爽, 王宁宜, 杭青青, 张宇辰, 石枫. 邻羟基苯基取代的对亚甲基苯醌参与的催化不对称反应研究进展★[J]. 化学学报, 2023, 81(7): 793-808. |
| [3] | 袁芳艳, 李超, 罗美明, 曾小明. 铬催化酮羰基的脱氧偶联反应合成四取代烯烃★[J]. 化学学报, 2023, 81(5): 456-460. |
| [4] | 韩明亮, 徐丽华. 过渡金属催化的硫酯的交叉偶联反应研究进展[J]. 化学学报, 2023, 81(4): 381-392. |
| [5] | 徐清浩, 魏立谱, 张震, 肖斌. 铜促进的锗亲电试剂与烷基溴合成四烷基锗※[J]. 化学学报, 2022, 80(4): 428-431. |
| [6] | 邓卓基, 欧阳溢凡, 敖运林, 蔡倩. 铜催化不对称去对称化分子内烯基C—N偶联反应[J]. 化学学报, 2021, 79(5): 649-652. |
| [7] | 汪欣, 张贤睿, 黄宗煜, 樊新元, 陈鹏. 生物正交反应在我国的研究进展[J]. 化学学报, 2021, 79(4): 406-413. |
| [8] | 朱雪敏, 白小燕, 王海峰, 胡平, 汪必琴, 赵可清. 苯并䓛盘状液晶: 合成、柱状相和光物理性质[J]. 化学学报, 2021, 79(12): 1486-1493. |
| [9] | 袁宏宇, 徐敏敏, 姚建林. 电化学SPR协同催化对氯苯硫酚界面反应的SERS研究[J]. 化学学报, 2021, 79(12): 1481-1485. |
| [10] | 李翼, 徐明华. 不对称Petasis反应在手性胺类化合物合成中的应用[J]. 化学学报, 2021, 79(11): 1345-1359. |
| [11] | 董奎, 刘强, 吴骊珠. 放氢交叉偶联反应[J]. 化学学报, 2020, 78(4): 299-310. |
| [12] | 程磊, 周其林. 镍催化构筑C(sp3)—C(sp3)键反应研究进展[J]. 化学学报, 2020, 78(10): 1017-1029. |
| [13] | 刘玉成, 郑啸, 黄培强. 光催化氧化还原体系中硝酮与芳香叔胺的自由基偶联反应[J]. 化学学报, 2019, 77(9): 850-855. |
| [14] | 刘茹雪, 何小燕, 牛力同, 吕柏霖, 余菲, 张哲, 杨志旺. 具有分级纳米结构的In2S3/CdIn2S4在可见光下催化苯甲胺的氧化偶联反应[J]. 化学学报, 2019, 77(7): 653-660. |
| [15] | 李月, 姜宇晨, 蒋平平, 杜盛郁, 姜就胜, 冷炎. 多孔碳球封装纳米碳化钼催化剂无溶剂催化苄胺偶联反应[J]. 化学学报, 2019, 77(1): 66-71. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||