化学学报 ›› 2024, Vol. 82 ›› Issue (4): 426-434.DOI: 10.6023/A24010019 上一篇 下一篇
研究论文
宋瑞, 赵铭钦, 王帅, 卢垚, 鲍晓冰, 罗巧梅, 苟蕾, 樊小勇*( ), 李东林
), 李东林
投稿日期:2024-01-18
发布日期:2024-03-01
基金资助:
Rui Song, Mingqin Zhao, Shuai Wang, Yao Lu, Xiaobing Bao, Qiaomei Luo, Lei Gou, Xiaoyong Fan*( ), Donglin Li
), Donglin Li
Received:2024-01-18
Published:2024-03-01
Contact:
* E-mail: Supported by:文章分享

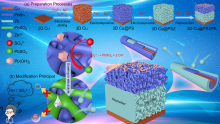
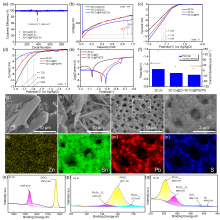
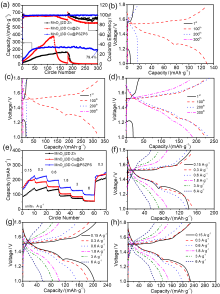
锌离子电池具有能量密度高、安全性好、价格低廉等优点, 被认为是规模化储能的理想选择之一. 然而, 其锌负极在电化学循环过程中易产生枝晶、析氢和表面钝化等, 造成电池库伦效率低、锌利用率低和循环寿命短等缺陷, 限制了其规模化应用. 本工作采用电沉积法在自制三维多孔铜内先沉积具有高亲锌性的PbSn合金层, 再沉积Zn层, 最后通过化学置换, 在上层孔表面获得PbSn合金层, 其在ZnSO4电解液中形成较低亲锌性的PbSO4层, 最终形成由三维多孔内部到外部亲锌性逐渐降低的具有三维亲锌性梯度的锌电极3D Cu@PbSn@Zn@PbSn (3D Cu@PSZPS). 三维多孔结构可提高电极比表面积, 提供更均匀的电场分布, 降低电流密度, 提高锌沉积容量, 抑制枝晶生长、析氢和钝化; 内层孔PbSn合金具有高亲锌性, 可诱导锌从内层孔沉积, 抑制锌枝晶生长; Pb和Sn具有高析氢过电位, 可抑制析氢, 同时抑制由于析氢致使电极表面pH值升高而产生的钝化. 因此该电极结构可获得优异的电化学性能, 在电流密度5 mA•cm-2, 面积容量1 mAh•cm-2的条件下, 可实现锌的稳定沉积/剥离900次以上, 首圈成核过电位仅16.4 mV, 首圈库伦效率高达99.66%; 以此电极组装的对称电池可以稳定循环超过700 h; 采用商业MnO2与之匹配得到的MnO2||3D Cu@PSZPS全电池在0.3 A•g-1时具有238.7 mAh•g-1的可逆比容量和300次循环后79.4%的容量保持率, 在1.8 A•g-1的电流密度下可稳定循环2000次以上.
宋瑞, 赵铭钦, 王帅, 卢垚, 鲍晓冰, 罗巧梅, 苟蕾, 樊小勇, 李东林. 三维多孔结构和亲锌性梯度协同构筑无枝晶锌电极[J]. 化学学报, 2024, 82(4): 426-434.
Rui Song, Mingqin Zhao, Shuai Wang, Yao Lu, Xiaobing Bao, Qiaomei Luo, Lei Gou, Xiaoyong Fan, Donglin Li. Three-Dimensional Porous Structure and Zincophile Gradient Enabling Dendrite Free Zinc Anode[J]. Acta Chimica Sinica, 2024, 82(4): 426-434.







| [1] |
Zampardi, G.; La Mantia, F. Nat. Commun. 2022, 13, 687.
doi: 10.1038/s41467-022-28381-x pmid: 35115524 |
| [2] |
Li, X. Y.; Wang, L.; Fu, Y.-H.; Dang, H.; Wang, D. H.; Ran, F. Nano Energy 2023, 116, 108858.
doi: 10.1016/j.nanoen.2023.108858 |
| [3] |
Yang, J.; Yin, B.; Sun, Y.; Pan, H.; Sun, W.; Jia, B.; Zhang, S.; Ma, T. Nano-Micro Lett. 2022, 14, 42.
|
| [4] |
Wang, K. X.; Fan, X. Y.; Chen, S. J.; Deng, J. K.; Zhang, L. L.; Jing, M. S.; Li, J. L.; Gou, L.; Li, D. L.; Ma, Y. Small 2022, 19, 2206287.
doi: 10.1002/smll.v19.8 |
| [5] |
Zhao, X. Y.; Liang, X. Q.; Li, Y.; Chen, Q. G.; Chen, M. H. Energy Storage Mater. 2021, 42, 533.
|
| [6] |
Zhang, Y.; Zheng, X.; Wang, N.; Lai, W. H.; Liu, Y.; Chou, S. L.; Liu, H. K.; Dou, S. X.; Wang, Y. X. Chem. Sci. 2022, 13, 14246.
doi: 10.1039/D2SC04945G |
| [7] |
Zhou, L. F.; Du, T.; Li, J. Y.; Wang, Y. S.; Gong, H.; Yang, Q. R.; Chen, H.; Luo, W. B.; Wang, J. Z. Mater. Horiz. 2022, 9, 2722.
doi: 10.1039/d2mh00973k pmid: 36196916 |
| [8] |
Qin, R. Z.; Wang, Y. T.; Yao, L.; Yang, L. Y.; Zhao, Q. H.; Ding, S. X.; Liu, L. L.; Pan, F. Nano Energy 2022, 98, 107333.
doi: 10.1016/j.nanoen.2022.107333 |
| [9] |
Zhu, Y. Z.; Guan, P. Y.; Zhu, R. B.; Zhang, S.; Feng, Z. H.; Li, M. Y.; Wan, T.; Hu, L.; Liu, Y. J.; Li, Q.; Yu, J.; Chu, D. W. J. Energy Chem. 2023, 87, 61.
doi: 10.1016/j.jechem.2023.08.024 |
| [10] |
Zhou, M.; Guo, S.; Fang, G. Z.; Sun, H. M.; Cao, X. X.; Zhou, J.; Pan, A. Q.; Liang, S. Q. J. Energy Chem. 2021, 55, 549.
doi: 10.1016/j.jechem.2020.07.021 |
| [11] |
Wang, C.; Wang, D. D.; Lv, D.; Peng, H. L.; Song, X. X.; Yang, J.; Qian, Y. T. Adv. Energy Mater. 2023, 13, 2204388.
doi: 10.1002/aenm.v13.20 |
| [12] |
Lu, H. Y.; Liu, L. Y.; Zhang, J. K.; Xu, J. T. J. Colloid Interface Sci. 2022, 617, 422.
doi: 10.1016/j.jcis.2022.03.010 |
| [13] |
Cui, Y.; Zhao, Q.; Wu, X.; Chen, X.; Yang, J.; Wang, Y.; Qin, R.; Ding, S.; Song, Y.; Wu, J.; Yang, K.; Wang, Z.; Mei, Z.; Song, Z.; Wu, H.; Jiang, Z.; Qian, G.; Yang, L.; Pan, F. Angew. Chem., Int. Ed. 2020, 59, 16594.
doi: 10.1002/anie.v59.38 |
| [14] |
Fan, X. Y.; Yang, H.; Feng, B.; Zhu, Y. Q.; Wu, Y.; Sun, R. B.; Gou, L.; Xie, J.; Li, D. L.; Ding, Y. L. Chem. Eng. J. 2022, 445, 136799.
doi: 10.1016/j.cej.2022.136799 |
| [15] |
Ji, H. M.; Xie, C. L.; Zhang, Q.; Li, Y. X.; Li, H. H.; Wang, H. Y. Acta Chim. Sinica 2023, 81, 29. (in Chinese)
doi: 10.6023/A22100413 |
|
(姬慧敏, 谢春霖, 张旗, 李熠鑫, 李欢欢, 王海燕, 化学学报, 2023, 81, 29.)
doi: 10.6023/A22100413 |
|
| [16] |
Liu, B.; Wang, S.; Wang, Z.; Lei, H.; Chen, Z.; Mai, W. Small 2020, 16, 2001323.
doi: 10.1002/smll.v16.22 |
| [17] |
Yang, J. L.; Yang, P. H.; Yan, W. Q.; Zhao, J. W.; Fan, H. J. Energy Storage Mater. 2022, 51, 259.
|
| [18] |
Yin, Y.; Wang, S.; Zhang, Q.; Song, Y.; Chang, N.; Pan, Y.; Zhang, H.; Li, X. Adv. Mater. 2020, 32, 1906803.
doi: 10.1002/adma.v32.6 |
| [19] |
Li, Y. M.; Guo, Q.; Wu, Y.; Ying, D. F.; Yu, Y. N.; Chi, T. S.; Xia, S. J.; Zhou, X. F.; Liu, Z. P. Adv. Funct. Mater. 2023, 33, 2214523.
doi: 10.1002/adfm.v33.19 |
| [20] |
Zhao, R.; Dong, X.; Liang, P.; Li, H.; Zhang, T.; Zhou, W.; Wang, B.; Yang, Z.; Wang, X.; Wang, L.; Sun, Z.; Bu, F.; Zhao, Z.; Li, W.; Zhao, D.; Chao, D. Adv. Mater. 2023, 35, 2209288.
doi: 10.1002/adma.v35.17 |
| [21] |
Zhao, Z. M.; Lai, J.; Ho, D. T.; Lei, Y. J.; Yin, J.; Chen, L.; Schwingenschlögl, U.; Alshareef, H. N. ACS Energy Lett. 2022, 8, 608.
doi: 10.1021/acsenergylett.2c02520 |
| [22] |
Wang, Y. H.; Zhao, R.; Liu, M. Q.; Yang, J. J.; Zhang, A. Q.; Yue, J. S.; Wu, C.; Bai, Y. Adv. Energy Mater. 2023, 13, 2302707.
doi: 10.1002/aenm.v13.43 |
| [23] |
Kang, L.; Zheng, J.; Yue, K.; Yuan, H.; Luo, J.; Wang, Y.; Liu, Y.; Nai, J.; Tao, X. Small 2023, 19, 2304094.
doi: 10.1002/smll.v19.44 |
| [24] |
Shi, X.; Xie, J. H.; Wang, J.; Xie, S. L.; Yang, Z. J.; Lu, X. H. Nat. Commun. 2024, 15, 302.
doi: 10.1038/s41467-023-44615-y |
| [25] |
Shen, F.; Du, H.; Qin, H.; Wei, Z.; Kuang, W.; Hu, N.; Lv, W.; Yi, Z.; Huang, D.; Chen, Z.; He, H. Small 2023, 25, 2305119.
|
| [26] |
Zhu, K. P.; Wu, L. Y.; Guo, C.; Pu, J.; Liu, Y.; Chen, X.; Chen, Y. T.; Xue, P.; Han, J.; Yao, Y. G. Adv. Funct. Mater. 2023, 33, 2305098.
doi: 10.1002/adfm.v33.47 |
| [27] |
Gao, Y.; Cao, Q.; Pu, J.; Zhao, X.; Fu, G.; Chen, J.; Wang, Y.; Guan, C. Adv. Mater. 2023, 35, 2207573.
doi: 10.1002/adma.v35.6 |
| [28] |
Li, J.; Zou, P. C.; Chiang, S. W.; Yao, W. T.; Wang, Y.; Liu, P.; Liang, C. W.; Kang, F. Y.; Yang, C. Energy Storage Mater. 2020, 24, 700.
|
| [29] |
Cao, Q.; Gao, Y.; Pu, J.; Zhao, X.; Wang, Y.; Chen, J.; Guan, C. Nat. Commun. 2023, 14, 641.
doi: 10.1038/s41467-023-36386-3 |
| [30] |
Fan, X. Y.; Yang, H.; Wang, X. X.; Han, J. X.; Wu, Y.; Gou, L.; Li, D. L.; Ding, Y. L. Adv. Mater. Interfaces 2021, 8, 2002184.
doi: 10.1002/admi.v8.7 |
| [31] |
Liang, G. J.; Zhu, J. X.; Yan, B. X.; Li, Q.; Chen, A.; Chen, Z.; Wang, X. Q.; Xiong, B.; Fan, J.; Xu, J.; Zhi, C. Y. Energy Environ. Sci. 2022, 15, 1086.
doi: 10.1039/D1EE03749H |
| [32] |
Shen, Z. X.; Luo, L.; Li, C. W.; Pu, J.; Xie, J. P.; Wang, L. T.; Huai, Z.; Dai, Z. Y.; Yao, Y. G.; Hong, G. Adv. Energy Mater. 2021, 11, 2100214.
doi: 10.1002/aenm.v11.16 |
| [33] |
Wang, S.; Fan, X. Y.; Cui, Y.; Gou, L.; Wang, X. G.; Li, D. Acta Chim. Sinica 2019, 77, 551. (in Chinese)
doi: 10.6023/A19020057 |
|
(王珊, 樊小勇, 崔宇, 苟蕾, 王新刚, 李东林, 化学学报, 2019, 77, 551.)
doi: 10.6023/A19020057 |
|
| [34] |
Tao, F.; Liu, Y.; Ren, X. Y.; Wang, J.; Zhou, Y. Z.; Miao, Y. J.; Ren, F. Z.; Wei, S. Z.; Ma, J. M. J. Energy Chem. 2022, 66, 397.
doi: 10.1016/j.jechem.2021.08.022 |
| [35] |
Fan, X. Y.; Zhu, Y. Q.; Wu, Y.; Zhang, S.; Xu, L.; Gou, L.; Li, D. L. Chem. J. Chin. Univ. 2022, 43, 65. (in Chinese)
|
|
(樊小勇, 朱永强, 毋妍, 张帅, 许磊, 苟蕾, 李东林, 高等学校化学学报, 2022, 43, 65.)
|
|
| [36] |
Wu, Y. C.; Du, H.; Zhu, J. X.; Xu, N.; Zhou, L.; Mai, L. Q. Chem. J. Chin. Univ. 2023, 44, 61. (in Chinese)
|
|
(吴育才, 杜寰, 朱杰鑫, 许诺, 周亮, 麦立强, 高等学校化学学报, 2023, 44, 61.)
|
|
| [37] |
Xu, J.; Li, X. G.; Wang, Z. L.; Dong, C. F. Chin. J. of Nonferrous Met. 2004, 14, 1217. (in Chinese)
|
|
(徐璟, 李晓刚, 王泽力, 董超芳, 中国有色金属学报, 2004, 14, 1217.)
|
|
| [38] |
Li, H.; Guo, C.; Zhang, T.; Xue, P.; Zhao, R.; Zhou, W.; Li, W.; Elzatahry, A.; Zhao, D.; Chao, D. Nano Lett. 2022, 22, 4223.
doi: 10.1021/acs.nanolett.2c01235 |
| [39] |
Ruan, P.; Chen, X.; Qin, L.; Tang, Y.; Lu, B.; Zeng, Z.; Liang, S.; Zhou, J. Adv. Mater. 2023, 35, 2300577.
doi: 10.1002/adma.v35.31 |
| [40] |
Pan, H. L.; Shao, Y. Y.; Yan, P. F.; Cheng, Y. W.; Han, K. S.; Nie, Z. M.; Wang, C. M.; Yang, J. H.; Li, X. L.; Bhattacharya, P.; Mueller, K. T.; Liu, J. Nat. Energy 2016, 1, 16039.
doi: 10.1038/nenergy.2016.39 |
| [41] |
Fan, X. Y.; Zhang, S.; Zhu, Y. Q.; Jing, M. S.; Wang, K. X.; Zhang, L. L.; Li, J. L.; Xu, L.; Gou, L.; Li, D. L. Acta Chim. Sinica 2022, 80, 517. (in Chinese)
doi: 10.6023/A21110529 |
|
(樊小勇, 张帅, 朱永强, 敬茂森, 王凯鑫, 张露露, 李巨龙, 许磊, 苟蕾, 李东林, 化学学报, 2022, 80, 517.)
doi: 10.6023/A21110529 |
| [1] | 郝再涛, 赵健飞, 李慧同, 李展, 潘朗, 李江. 亲锌聚阴离子交联聚合物薄膜与铜刻蚀协同稳定锌负极[J]. 化学学报, 2024, 82(4): 416-425. |
| [2] | 鲍梦凡, 陈诗洁, 邵霞, 邓慧娟, 冒爱琴, 檀杰. 低共熔溶剂辅助制备空心球状钙钛矿型高熵氧化物及高倍率储锂性能[J]. 化学学报, 2024, 82(3): 303-313. |
| [3] | 贾洋刚, 陈诗洁, 邵霞, 程婕, 林娜, 方道来, 冒爱琴, 李灿华. 高性能无钴化钙钛矿型高熵氧化物负极材料的制备及储锂性能研究[J]. 化学学报, 2023, 81(5): 486-495. |
| [4] | 张雅岚, 苑志祥, 张浩, 张建军, 崔光磊. 高镍三元高比能固态锂离子电池的研究进展[J]. 化学学报, 2023, 81(12): 1724-1738. |
| [5] | 刘稳, 王昱捷, 杨慧琴, 李成杰, 吴娜, 颜洋. 离子液体非共价诱导制备碳纳米管/石墨烯集流体用于钠金属负极[J]. 化学学报, 2023, 81(10): 1379-1386. |
| [6] | 姬慧敏, 谢春霖, 张旗, 李熠鑫, 李欢欢, 王海燕. 水系锌离子电池负极集流体关键问题及设计策略[J]. 化学学报, 2023, 81(1): 29-41. |
| [7] | 张国强, 霍京浩, 王鑫, 郭守武. 基于P掺杂TiO2/C纳米管负极的高性能锂离子电容器[J]. 化学学报, 2023, 81(1): 6-13. |
| [8] | 梁世硕, 康树森, 杨东, 胡建华. 锂金属负极界面修饰及其在硫化物全固态电池中的应用[J]. 化学学报, 2022, 80(9): 1264-1268. |
| [9] | 毕文超, 张琳锋, 陈健, 田瑞雪, 黄昊, 姚曼. 单斜ZnP2负极材料的锂化机理及性能[J]. 化学学报, 2022, 80(6): 756-764. |
| [10] | 樊小勇, 张帅, 朱永强, 敬茂森, 王凯鑫, 张露露, 李巨龙, 许磊, 苟蕾, 李东林. 三维多孔铜和锌镀层协同构筑无枝晶锂金属电极[J]. 化学学报, 2022, 80(4): 517-525. |
| [11] | 庄容, 许潇洒, 曲昌镇, 徐顺奇, 于涛, 王洪强, 徐飞. 多孔聚合物在锂金属负极保护中的研究进展[J]. 化学学报, 2021, 79(4): 378-387. |
| [12] | 张璐, 王文凤, 张洪明, 韩树民, 王利民. 水系锌离子电池研究进展和挑战[J]. 化学学报, 2021, 79(2): 158-175. |
| [13] | 李燕丽, 于丹丹, 林森, 孙东飞, 雷自强. α-MnO2纳米棒/多孔碳正极材料的制备及水系锌离子电池性能研究[J]. 化学学报, 2021, 79(2): 200-207. |
| [14] | 常智, 乔羽, 杨慧军, 邓瀚, 朱星宇, 何平, 周豪慎. 金属有机框架(MOFs)材料在锂离子电池及锂金属电池电解液中的应用[J]. 化学学报, 2021, 79(2): 139-145. |
| [15] | 董瑞琪, 吴锋, 白莹, 吴川. 钠离子电池硬碳负极储钠机理及优化策略[J]. 化学学报, 2021, 79(12): 1461-1476. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||