化学学报 ›› 2024, Vol. 82 ›› Issue (4): 416-425.DOI: 10.6023/A24010006 上一篇 下一篇
研究论文
投稿日期:2024-01-08
发布日期:2024-04-02
基金资助:
Zaitao Hao, Jianfei Zhao, Huitong Li, Zhan Li, Lang Pan, Jiang Li*( )
)
Received:2024-01-08
Published:2024-04-02
Contact:
* E-mail: Supported by:文章分享
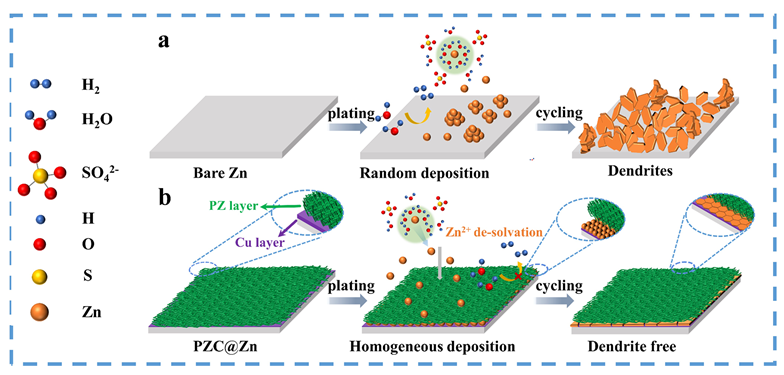
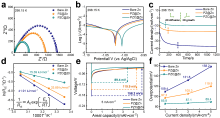
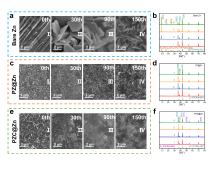
可充电水系锌基电池具有高容量、高能量密度、低氧化还原电位、低成本、安全性好等优点, 被认为是一种可替代的能源存储器件. 然而, 在镀锌/剥离过程中, 锌金属负极不可控的枝晶生长和复杂的副反应极大地降低了库伦效率, 其可逆性较差, 阻碍锌基电池的实际应用. 在此, 开发了一种简单、可控且有效的方法, 首先利用电化学沉积法在锌箔上沉积了一种由聚苯乙烯磺酸钠(PSS)与氯化锌反应得到孔道丰富的聚阴离子交联聚合物薄膜(记为PZ, 其中P代表PSS、Z代表ZnCl2), 然后再利用置换反应引入亲锌的化学惰性金属铜, 得到亲锌聚阴离子交联聚合物与铜刻蚀保护层(记为PZC, 其中C代表Cu). PZC@Zn具有丰富的磺酸根官能团促进[Zn(H2O)6]2+脱溶, 提高Zn2+界面传输速率, 排斥SO42-与锌负极接触. 通过重建铜刻蚀层, 铜的高亲锌性促进了沉积动力学, 铜的化学惰性抑制了副反应的发生. 结果表明, 在5 mA•cm-2的高电流密度下, PZC@Zn//PZC@Zn对称电池寿命高达4055 h(比裸锌对称电池提高了31倍), 累积电镀容量达到10.14 Ah•cm-2, Ti//PZC@Zn半电池库伦效率(CE)为98.28%, 可以实现稳定且可逆的镀锌/剥离. YP-50F//PZC@Zn锌离子混合超级电容器在2 A•g-1下稳定循环15000次, 放电比容量高达82.35 mAh•g-1. α-MnO2//PZC@Zn水系锌离子电池在1 A•g-1下循环2000次后放电比容量高达103.57 mAh•g-1, CE为99.58%. 这项工作为设计先进的无枝晶锌金属负极提供了一种新方法.
郝再涛, 赵健飞, 李慧同, 李展, 潘朗, 李江. 亲锌聚阴离子交联聚合物薄膜与铜刻蚀协同稳定锌负极[J]. 化学学报, 2024, 82(4): 416-425.
Zaitao Hao, Jianfei Zhao, Huitong Li, Zhan Li, Lang Pan, Jiang Li. Stable Zinc Anodes through Synergistic Copper Etching and Zincophilic Polyanionic Crosslinking Membrane[J]. Acta Chimica Sinica, 2024, 82(4): 416-425.












| [1] |
Chen, J. Acta Chim. Sinica 2017, 75, 127. (in Chinese)
doi: 10.6023/A1702E001 |
|
(陈军, 化学学报, 2017, 75, 127.)
|
|
| [2] |
Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K. S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; Mueller, K. T.; Liu, J. Nat. Energy. 2016, 1, 1.
doi: 10.1038/ng0492-1 |
| [3] |
Cui, M.; Xiao, Y.; Kang, L.; Du, W.; Gao, Y.; Sun, X.; Zhou, Y.; Li, X.; Li, H.; Jiang, F.; Zhi, C. ACS Appl. Energy Mater. 2019, 2, 6490.
doi: 10.1021/acsaem.9b01063 |
| [4] |
Zhang, Q.; Luan, J.; Tang, Y.; Ji, X.; Wang, H. Angew. Chem. Int. Ed. 2020, 59, 13180.
doi: 10.1002/anie.202000162 pmid: 32124537 |
| [5] |
Yang, J. L.; Li, J.; Zhao, J. W.; Liu, K.; Yang, P.; Fan, H. J. Adv. Mater. 2022, 34, 2022382.
|
| [6] |
Ji, H.-M.; Xie, C.-L.; Zhang, Q.; Li, Y.-X.; Li, H.-H.; Wang, H.-Y. Acta Chim. Sinica 2023, 81, 29. (in Chinese)
doi: 10.6023/A22100413 |
|
(姬慧敏, 谢春霖, 张旗, 李熠鑫, 李欢欢, 王海燕, 化学学报, 2023, 81, 29.)
doi: 10.6023/A22100413 |
|
| [7] |
Dong, Y.; Miao, L.; Ma, G.; Di, S.; Wang, Y.; Wang, L.; Xu, J.; Zhang, N. Chem. Sci. 2021, 12, 5843.
doi: 10.1039/D0SC06734B |
| [8] |
Song, X.; He, H.; Aboonasr Shiraz, M. H.; Zhu, H.; Khosrozadeh, A.; Liu, J. Chem. Commun. 2021, 57, 1246.
doi: 10.1039/D0CC06076C |
| [9] |
Cao, L.; Li, D.; Hu, E.; Xu, J.; Deng, T.; Ma, L.; Wang, Y.; Yang, X.-Q.; Wang, C. J. Am. Chem. Soc. 2020, 142, 21404.
doi: 10.1021/jacs.0c09794 |
| [10] |
Cao, L.; Li, D.; Pollard, T.; Deng, T.; Zhang, B.; Yang, C.; Chen, L.; Vatamanu, J.; Hu, E.; Hourwitz, M. J.; Ma, L.; Ding, M.; Li, Q.; Hou, S.; Gaskell, K.; Fourkas, J. T.; Yang, X.-Q.; Xu, K.; Borodin, O.; Wang, C. Nat. Nanotechnol. 2021, 16, 902.
doi: 10.1038/s41565-021-00905-4 |
| [11] |
Zhu, Y.; Yin, J.; Zheng, X.; Emwas, A.-H.; Lei, Y.; Mohammed, O. F.; Cui, Y.; Alshareef, H. N. Energy Environ. Sci. 2021, 14, 4463.
doi: 10.1039/D1EE01472B |
| [12] |
Han, J.; Mariani, A.; Varzi, A.; Passerini, S. J. Power Sources 2021, 485, 229329.
doi: 10.1016/j.jpowsour.2020.229329 |
| [13] |
Guo, W.; Cong, Z.; Guo, Z.; Chang, C.; Liang, X.; Liu, Y.; Hu, W.; Pu, X. Energy Storage Mater. 2020, 30, 104.
|
| [14] |
Zhou, Y.; Wang, X.; Shen, X.; Shi, Y.; Zhu, C.; Zeng, S.; Xu, H.; Cao, P.; Wang, Y.; Di, J.; Li, Q. J. Mater. Chem. A 2020, 8, 11719.
doi: 10.1039/D0TA02791J |
| [15] |
Li, S.; Fu, J.; Miao, G.; Wang, S.; Zhao, W.; Wu, Z.; Zhang, Y.; Yang, X. Adv. Mater. 2021, 33, 2008424.
doi: 10.1002/adma.v33.21 |
| [16] |
Cai, Z.; Ou, Y.; Wang, J.; Xiao, R.; Fu, L.; Yuan, Z.; Zhan, R.; Sun, Y. Energy Storage Mater. 2020, 27, 205.
|
| [17] |
Wang, Y.; Chen, Y.; Liu, W.; Ni, X.; Qing, P.; Zhao, Q.; Wei, W.; Ji, X.; Ma, J.; Chen, L. J. Mater. Chem. A 2021, 9, 8452.
doi: 10.1039/D0TA12177K |
| [18] |
Dong, L.; Yang, W.; Yang, W.; Tian, H.; Huang, Y.; Wang, X.; Xu, C.; Wang, C.; Kang, F.; Wang, G. Chem. Eng. J. 2020, 384, 123355.
doi: 10.1016/j.cej.2019.123355 |
| [19] |
Xia, A.; Pu, X.; Tao, Y.; Liu, H.; Wang, Y. Appl. Surf. Sci. 2019, 481, 852.
doi: 10.1016/j.apsusc.2019.03.197 |
| [20] |
Anand, A.; Ji Dixit, R.; Verma, A.; Basu, S. Energy Technol. 2023, 2300698.
|
| [21] |
Jiao, S.; Fu, J.; Wu, M.; Hua, T.; Hu, H. ACS Nano 2021, 16, 1013.
doi: 10.1021/acsnano.1c08638 |
| [22] |
Liu, M.-C.; Tian, C.-Y.; Zhang, D.-T.; Zhang, Y.-S.; Zhang, B.-M.; Wang, Y.-Y.; Li, C.-Y.; Liu, M.-J.; Gu, B.; Zhao, K.; Kong, L.-B.; Chueh, Y.-L. Nano Energy 2022, 103, 107805.
doi: 10.1016/j.nanoen.2022.107805 |
| [23] |
Liu, X.; Han, Q.; Ma, Q.; Wang, Y.; Liu, C. Small 2022, 18, 2203327.
doi: 10.1002/smll.v18.39 |
| [24] |
Zhao, K.; Wang, C.; Yu, Y.; Yan, M.; Wei, Q.; He, P.; Dong, Y.; Zhang, Z.; Wang, X.; Mai, L. Adv. Mater. Interfaces 2018, 5, 1800848.
|
| [25] |
Labriola, L.; Jadoul, M. Nephrol. Dial. Transplant. 2020, 35, 1455.
doi: 10.1093/ndt/gfaa004 |
| [26] |
Kwak, J. C. T.; Nelson, R. W. P. J. Phys. Chem. 1978, 82, 2388.
doi: 10.1021/j100511a008 |
| [27] |
Zhou, J.; Xie, M.; Wu, F.; Mei, Y.; Hao, Y.; Huang, R.; Wei, G.; Liu, A.; Li, L.; Chen, R. Adv. Mater. 2021, 33, 2101649.
doi: 10.1002/adma.v33.33 |
| [28] |
Zou, P.; Nykypanchuk, D.; Doerk, G.; Xin, H. L. ACS Appl. Mater. Interfaces 2021, 13, 60092.
doi: 10.1021/acsami.1c20995 |
| [29] |
Wang, H.; Chen, Y.; Yu, H.; Liu, W.; Kuang, G.; Mei, L.; Wu, Z.; Wei, W.; Ji, X.; Qu, B.; Chen, L. Adv. Funct. Mater. 2022, 32, 2205600.
doi: 10.1002/adfm.v32.43 |
| [30] |
Liang, S.; Sui, G.; Li, J.; Guo, D.; Luo, Z.; Xu, R.; Yao, H.; Wang, C.; Chen, S. Int. J. Hydrogen Energy 2022, 47, 11190.
doi: 10.1016/j.ijhydene.2022.01.154 |
| [31] |
Su, Y.; Li, S.; He, D.; Yu, D.; Liu, F.; Shao, N.; Zhang, Z. ACS Sustainable Chem. Eng. 2018, 6, 11989.
doi: 10.1021/acssuschemeng.8b02287 |
| [32] |
Fan, L.; Li, Z.; Kang, W.; Cheng, B. Renewable Energy 2020, 155, 309.
doi: 10.1016/j.renene.2020.03.153 |
| [33] |
Zhang, Q.; Luan, J.; Huang, X.; Zhu, L.; Tang, Y.; Ji, X.; Wang, H. Small 2020, 16, 2000929.
doi: 10.1002/smll.v16.35 |
| [34] |
Liang, P.; Yi, J.; Liu, X.; Wu, K.; Wang, Z.; Cui, J.; Liu, Y.; Wang, Y.; Xia, Y.; Zhang, J. Adv. Funct. Mater. 2020, 30, 1908528.
doi: 10.1002/adfm.v30.13 |
| [35] |
Lu, H.; Liu, L.; Zhang, J.; Xu, J. J. Colloid Interface Sci. 2022, 617, 422.
doi: 10.1016/j.jcis.2022.03.010 |
| [36] |
Zhao, Z.; Wang, R.; Peng, C.; Chen, W.; Wu, T.; Hu, B.; Weng, W.; Yao, Y.; Zeng, J.; Chen, Z.; Liu, P.; Liu, Y.; Li, G.; Guo, J.; Lu, H.; Guo, Z. Nat. Commun. 2021, 12, 6606.
doi: 10.1038/s41467-021-26947-9 |
| [37] |
Ma, L.; Li, Q.; Ying, Y.; Ma, F.; Chen, S.; Li, Y.; Huang, H.; Zhi, C. Adv. Mater. 2021, 33, 2007406.
doi: 10.1002/adma.v33.12 |
| [38] |
Sun, H.; Huyan, Y.; Li, N.; Lei, D.; Liu, H.; Hua, W.; Wei, C.; Kang, F.; Wang, J.-G. Nano Lett. 2023, 23, 1726.
doi: 10.1021/acs.nanolett.2c04410 |
| [39] |
Chen, T.; Wang, Y.; Yang, Y.; Huang, F.; Zhu, M.; Ang, B. T. W.; Xue, J. M. Adv. Funct. Mater. 2021, 31, 25495.
|
| [40] |
Xu, Y.; Wang, C.; Shi, Y.; Miao, G.; Fu, J.; Huang, Y. J. Mater. Chem. A 2021, 9, 25495.
doi: 10.1039/D1TA08400C |
| [41] |
Wang, Y.; Li, A.; Cheng, C. Mater. Today Chem. 2022, 26, 101057.
|
| [42] |
Li, Q.; Chen, A.; Wang, D.; Zhao, Y.; Wang, X.; Jin, X.; Xiong, B.; Zhi, C. Nat. Commun. 2022, 13, 3699.
doi: 10.1038/s41467-022-31461-7 |
| [43] |
Zhou, Y.; Xia, J.; Di, J.; Sun, Z.; Zhao, L.; Li, L.; Wu, Y.; Dong, L.; Wang, X.; Li, Q. Adv. Energy Mater. 2023, 13, 2203165.
doi: 10.1002/aenm.v13.10 |
| [44] |
Wang, Z.; Wang, Y.; Lin, Y.; Bian, G.; Liu, H.-Y.; Li, X.; Yin, J.; Zhu, J. ACS Appl. Mater. Interfaces 2022, 14, 47725.
doi: 10.1021/acsami.2c13030 |
| [1] | 何文, 王波, 冯晗俊, 孔祥如, 李桃, 肖睿. CO2捕集膜分离的Pebax基材料研究进展[J]. 化学学报, 2024, 82(2): 226-241. |
| [2] | 郭建荣, 张书玉, 贺军辉, 任世学. 基于生物质可降解薄膜的制备与应用[J]. 化学学报, 2024, 82(2): 242-256. |
| [3] | 伏成玉, 周星宇, 杨鹏. 基于蛋白质类淀粉样聚集的表面功能化★[J]. 化学学报, 2023, 81(11): 1566-1576. |
| [4] | 王洁, 叶雨晴, 李源, 马小杰, 王博. 基于无机纳米材料的抗菌抗病毒功能涂层和薄膜[J]. 化学学报, 2022, 80(9): 1338-1350. |
| [5] | 张琪, 江梦云, 刘天一, 曾意迅, 石胜伟. 可蒸镀自旋交叉配合物的薄膜与器件[J]. 化学学报, 2022, 80(9): 1351-1363. |
| [6] | 曹琳安, 魏敏. 电子导电金属有机框架薄膜的研究进展[J]. 化学学报, 2022, 80(7): 1042-1056. |
| [7] | 孙嘉贤, 刘禹廷, 尹志刚, 郑庆东. 基于吸收互补有机半导体本体复合薄膜的高性能柔性光突触晶体管[J]. 化学学报, 2022, 80(7): 936-945. |
| [8] | 闫彬, 薛丁江, 胡劲松. 硒化亚锗薄膜太阳能电池研究进展※[J]. 化学学报, 2022, 80(6): 797-804. |
| [9] | 张蒙茜, 冯霄. 共轭微孔聚合物膜的制备策略及其分离应用[J]. 化学学报, 2022, 80(2): 168-179. |
| [10] | 孙稷, 易玖琦, 程龙玖. 定向Monte Carlo格点搜索算法用于氧化铝团簇(Al2O3)n (n=1~50)的结构搜索[J]. 化学学报, 2021, 79(9): 1154-1163. |
| [11] | 董锦辉, 李进杰, 王赫, 刘彬秀, 彭博, 陈健壮, 林绍梁. 呼吸图法制备基于准聚轮烷的响应性薄膜[J]. 化学学报, 2021, 79(6): 803-808. |
| [12] | 吴峰, 苏倩倩, 周乐乐, 许鹏飞, 董傲, 钱卫平. 基于二氧化硅胶体晶体薄膜和反射干涉光谱的蛋白冠监测方法[J]. 化学学报, 2021, 79(3): 338-343. |
| [13] | 冯启琨, 张冬丽, 刘畅, 张涌新, 党智敏. 柔性高储能TPU/P(VDF-HFP)全有机复合薄膜的制备及性能表征[J]. 化学学报, 2021, 79(10): 1273-1280. |
| [14] | 杨英, 林飞宇, 朱从潭, 陈甜, 马书鹏, 罗媛, 朱刘, 郭学益. 无机钙钛矿太阳能电池稳定性研究进展[J]. 化学学报, 2020, 78(3): 217-231. |
| [15] | 王凯凯, 贺军辉. 基于季铵盐改性SiO2空心球的抗菌/减反增透双功能薄膜的制备和研究[J]. 化学学报, 2018, 76(10): 807-812. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||