化学学报 ›› 2025, Vol. 83 ›› Issue (8): 816-826.DOI: 10.6023/A25040102 上一篇 下一篇
研究论文
赵玉强a, 那迪a, 和晓波a, 朱立平b, 周莹b,*( ), 曾广智a,*(
), 曾广智a,*( ), 樊保敏a,*(
), 樊保敏a,*( )
)
投稿日期:2025-04-01
发布日期:2025-05-08
通讯作者:
周莹, 曾广智, 樊保敏
基金资助:
Yu-Qiang Zhaoa, Di Naa, Xiaobo Hea, Liping Zhub, Ying Zhoub,*( ), Guang-Zhi Zenga,*(
), Guang-Zhi Zenga,*( ), Baomin Fana,*(
), Baomin Fana,*( )
)
Received:2025-04-01
Published:2025-05-08
Contact:
Ying Zhou, Guang-Zhi Zeng, Baomin Fan
Supported by:文章分享
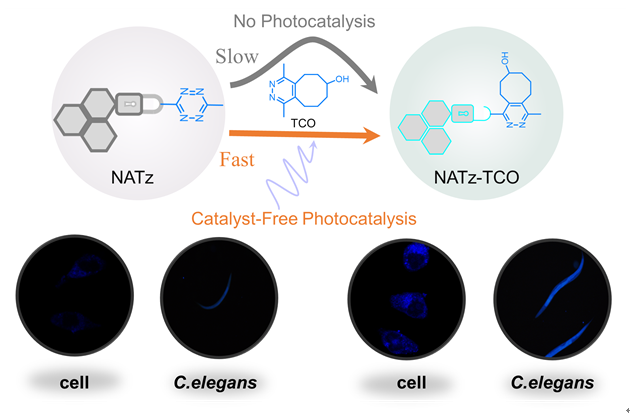
将具有生物正交反应活性的1,2,4,5-四氮嗪基团引入到1,8-萘酰亚胺荧光团上设计合成了光介导生物正交荧光探针NATz. 光谱测试显示, NATz与trans-cyclooctene (TCO)在磷酸盐缓冲液中反应后荧光被激活, 理论计算清晰解释了NATz的猝灭机制和荧光激活机制. 最重要的是, 在不加光催化剂的条件下, 365 nm紫外光显著提高了NATz与TCO的反应速率. 高分辨质谱和反应路径计算表明, 光对生物正交反应的促进效应是通过克服NATz与TCO之间的环加成反应能垒实现的. 细胞毒性测试证明了NATz具有良好的生物相容性, 在此基础上实现了细胞和多细胞生物(秀丽隐杆线虫)水平上的光介导生物正交成像. 最后, 基于NATz的结构, 引入吗啉基因设计并合成了具有溶酶体靶向能力的生物正交荧光探针Lyso-NATz. 一系列实验证明了Lyso-NATz在保持光介导生物正交荧光成像特性的同时, 实现了对溶酶体的靶向作用.
赵玉强, 那迪, 和晓波, 朱立平, 周莹, 曾广智, 樊保敏. 无需催化剂的光介导生物正交荧光探针实例[J]. 化学学报, 2025, 83(8): 816-826.
Yu-Qiang Zhao, Di Na, Xiaobo He, Liping Zhu, Ying Zhou, Guang-Zhi Zeng, Baomin Fan. A Catalyst-Free Example of Photomediated Biological Orthogonal Fluorescence Probes[J]. Acta Chimica Sinica, 2025, 83(8): 816-826.














| [1] |
|
|
(廖伊铭, 吴宝琪, 唐荣志, 化学进展, 2022, 34, 2134.)
doi: 10.7536/PC220103 |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
|
(章强, 黄文峻, 王延斌, 李兴建, 张宜恒, 化学进展, 2020, 32, 147.)
doi: 10.7536/PC190804 |
|
| [6] |
|
|
(王才, 周锋, 周剑, 有机化学, 2020, 40, 3065.)
doi: 10.6023/cjoc202005020 |
|
| [7] |
doi: 10.1039/d0sc03477k pmid: 34123155 |
| [8] |
|
| [9] |
|
| [10] |
|
|
(周远春, 周志, 杜玮, 陈应春, 化学学报, 2018, 76, 382.)
doi: 10.6023/A18040131 |
|
| [11] |
|
|
(吕健, 钟兴仁, 程津培, 罗三中, 化学学报, 2012, 70, 1518.)
doi: 10.6023/A12060346 |
|
| [12] |
|
|
(张贤睿, 罗惠鑫, 樊新元, 陈鹏, 中国科学: 化学, 2020, 50, 1280.)
|
|
| [13] |
doi: 10.1039/c7cs00184c pmid: 28660957 |
| [14] |
|
| [15] |
|
|
(方葛敏, 王晨, 石景, 郭庆祥, 化学学报, 2009, 67, 2335.)
|
|
| [16] |
|
|
(张洋子, 朱龙佼, 邵向丽, 许文涛, 生物技术通报, 2019, 35, 187.)
doi: 10.13560/j.cnki.biotech.bull.1985.2018-0096 |
|
| [17] |
|
| [18] |
|
|
(杨麦云, 陈鹏, 化学学报, 2015, 73, 783.)
doi: 10.6023/A15030214 |
|
| [19] |
doi: 10.6023/A20110530 |
|
(汪欣, 张贤睿, 黄宗煜, 樊新元, 陈鹏, 化学学报, 2021, 79, 406.)
doi: 10.6023/A20110530 |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
|
(粟敦妍, 李杰, 潘立立, 吴昊星, 毛梧宇, 药学学报, 2021, 56, 1086.)
|
|
| [26] |
|
|
(王晓蒙, 李杰, 沈国华, 潘立立, 田蓉, 孙洪宝, 吴昊星, 药学学报, 2020, 55, 1634.)
|
|
| [27] |
|
| [28] |
|
| [29] |
doi: 10.1021/jacs.2c10655 pmid: 36881718 |
| [30] |
doi: 10.1021/jacs.6b02168 pmid: 27078610 |
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
doi: 10.1002/anie.200906120 pmid: 20306505 |
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
doi: 10.1039/d0sc01009j pmid: 33384859 |
| [47] |
|
|
(徐萌萌, 蔡泉, 有机化学, 2022, 42, 698.)
doi: 10.6023/cjoc202109025 |
|
| [48] |
|
| [49] |
|
|
(杨聚梁, 戴俊, 娄筱叮, 夏帆, 科学通报, 2020, 65, 3497.)
|
|
| [50] |
|
| [51] |
|
| [52] |
|
| [1] | 刘童, 皮慧慧, 陈冰昆, 张小玲. 近红外二区硫系银量子点制备方法及癌症诊疗应用研究进展[J]. 化学学报, 2024, 82(9): 1001-1012. |
| [2] | 蒋励, 陈子晗, 凡勇. 近红外二区荧光探针在活体多重成像中的研究进展[J]. 化学学报, 2024, 82(10): 1069-1085. |
| [3] | 武虹乐, 郭锐, 迟涵文, 唐永和, 宋思睿, 葛恩香, 林伟英. 喹啉基粘度荧光探针的合成及其检测应用[J]. 化学学报, 2023, 81(8): 905-911. |
| [4] | 贺晓梦, 袁方, 张素雅, 张健健. 基于尼罗红类ONOO–近红外荧光探针的开发及其成像应用[J]. 化学学报, 2023, 81(11): 1515-1521. |
| [5] | 孙丽, 王亚静, 李涛, 郭英姝, 张书圣. 金纳米笼探针用于线粒体成像和光热损伤细胞★[J]. 化学学报, 2023, 81(10): 1301-1310. |
| [6] | 宋思睿, 唐永和, 孙良广, 郭锐, 姜冠帆, 林伟英. 基于香豆素荧光团的新型极性检测荧光探针的开发及其成像应用[J]. 化学学报, 2022, 80(9): 1217-1222. |
| [7] | 吴志芬, 柯建熙, 刘永升, 孙蓬明, 洪茂椿. 稀土近红外二区纳米荧光影像探针及其生物医学应用※[J]. 化学学报, 2022, 80(4): 542-552. |
| [8] | 汪欣, 张贤睿, 黄宗煜, 樊新元, 陈鹏. 生物正交反应在我国的研究进展[J]. 化学学报, 2021, 79(4): 406-413. |
| [9] | 李勇, 王栩, 解希雷, 张建, 唐波. 一氧化碳有机荧光探针和光控释放剂研究进展[J]. 化学学报, 2021, 79(1): 36-44. |
| [10] | 罗兴蕊, 陈敏文, 杨晴来. 近红外二区活体成像技术及其应用研究进展[J]. 化学学报, 2020, 78(5): 373-381. |
| [11] | 刘红文, 朱隆民, 娄霄峰, 袁林, 张晓兵. 用于细胞和组织中弗林蛋白酶特异性成像的双光子荧光探针研究[J]. 化学学报, 2020, 78(11): 1240-1245. |
| [12] | 贾伊祎, 王文杰, 梁玲, 袁荃. 核酸功能化稀土基纳米材料在生物检测中的应用[J]. 化学学报, 2020, 78(11): 1177-1184. |
| [13] | 郑斌, 程盛, 董华泽, 朱金苗, 韩钰, 杨亮, 胡进明. 一氧化氮响应性高分子荧光探针的构筑及其细胞内成像应用[J]. 化学学报, 2020, 78(10): 1089-1095. |
| [14] | 陈凯, 韩百川, 嵇思鑫, 孙瑾, 高振忠, 侯贤锋. 一种可用于直接检测空气中异氰酸酯的比率型荧光探针[J]. 化学学报, 2019, 77(4): 365-370. |
| [15] | 王少静, 李长伟, 李锦, 陈邦, 郭媛. 新型香豆素类氟离子荧光探针的合成及细胞成像研究[J]. 化学学报, 2017, 75(4): 383-390. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||