化学学报 ›› 2025, Vol. 83 ›› Issue (8): 810-815.DOI: 10.6023/A25050150 上一篇 下一篇
研究通讯
黄皓毅, 谢敏, 黄玉婷, 崔嘉豪, 蔡中正*( ), 朱剑波*(
), 朱剑波*( )
)
投稿日期:2025-05-09
发布日期:2025-06-17
通讯作者:
蔡中正, 朱剑波
作者简介:★ “中国青年化学家”专辑.
基金资助:
Haoyi Huang, Min Xie, Yuting Huang, Jiahao Cui, Zhongzheng Cai*( ), Jianbo Zhu*(
), Jianbo Zhu*( )
)
Received:2025-05-09
Published:2025-06-17
Contact:
Zhongzheng Cai, Jianbo Zhu
About author:★ For the VSI “Rising Stars in Chemistry”.
Supported by:文章分享
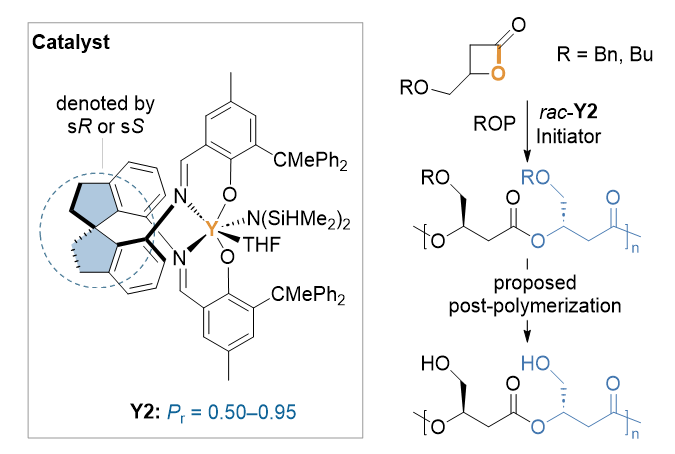
聚羟基脂肪酸酯(polyhydroxyalkanoates, PHAs)作为一类由细菌发酵制备的生物可降解材料, 因其性能可媲美商业聚烯烃, 被视为传统塑料的潜在替代品. 然而, 高昂的生产成本严重制约了其规模化应用. 尽管天然细菌发酵PHAs呈现丰富的结构多样性, 但产物多局限于烷基侧基取代衍生物. 本研究通过开发外消旋杂原子取代四元环内酯单体的立体选择性聚合体系, 成功合成了具有高间同立构结构的PHAs (Pr>0.95), 其分子结构显著区别于微生物合成PHAs, 突破了传统发酵法在结构调控上的局限性, 拓展了PHAs的结构多样性及潜在应用范围.
黄皓毅, 谢敏, 黄玉婷, 崔嘉豪, 蔡中正, 朱剑波. 螺环-salen配合物催化开环聚合制备立构规整、功能化聚羟基脂肪酸酯★[J]. 化学学报, 2025, 83(8): 810-815.
Haoyi Huang, Min Xie, Yuting Huang, Jiahao Cui, Zhongzheng Cai, Jianbo Zhu. Synthesis of Stereoregular and Functional Polyhydroxyalkanoates via Ring-Opening Polymerization Mediated by Spiro-salen Complexes★[J]. Acta Chimica Sinica, 2025, 83(8): 810-815.
| Entry | Monomer | Catalyst | Timeb | Conv.c/% | Mnd/kDa | Đd (Mw/Mn) | Pre |
|---|---|---|---|---|---|---|---|
| 1 | rac-BPLCH2OBu | rac-Y1 | 10 min 12 h | 17 51 | n.d.f 1.24 | n.d.f 1.27 | n.d.f 0.50 |
| 2 | rac-BPLCH2OBu | (R)-Y2 | 3.5 h | 73 | 7.59 | 1.07 | 0.66 |
| 3 | rac-BPLCH2OBu | rac-Y2 | 1 h | >99 | 8.26 | 1.12 | >0.95 |
| 4 | rac-BPLCH2OBn | rac-Y1 | 10 min 12 h | n.d.f 85 | n.d.f 1.06 | n.d.f 1.05 | n.d.f 0.65 |
| 5 | rac-BPLCH2OBn | (R)-Y1 | 6 h | 30 | n.d.f | n.d.f | 0.60 |
| 6 | rac-BPLCH2OBn | (R)-Y2 | 3.5 h | 79 | 8.50 | 1.15 | 0.49 |
| 7 | rac-BPLCH2OBn | rac-Y2 | 20 s | >99 | 10.7 | 1.13 | >0.95 |
| Entry | Monomer | Catalyst | Timeb | Conv.c/% | Mnd/kDa | Đd (Mw/Mn) | Pre |
|---|---|---|---|---|---|---|---|
| 1 | rac-BPLCH2OBu | rac-Y1 | 10 min 12 h | 17 51 | n.d.f 1.24 | n.d.f 1.27 | n.d.f 0.50 |
| 2 | rac-BPLCH2OBu | (R)-Y2 | 3.5 h | 73 | 7.59 | 1.07 | 0.66 |
| 3 | rac-BPLCH2OBu | rac-Y2 | 1 h | >99 | 8.26 | 1.12 | >0.95 |
| 4 | rac-BPLCH2OBn | rac-Y1 | 10 min 12 h | n.d.f 85 | n.d.f 1.06 | n.d.f 1.05 | n.d.f 0.65 |
| 5 | rac-BPLCH2OBn | (R)-Y1 | 6 h | 30 | n.d.f | n.d.f | 0.60 |
| 6 | rac-BPLCH2OBn | (R)-Y2 | 3.5 h | 79 | 8.50 | 1.15 | 0.49 |
| 7 | rac-BPLCH2OBn | rac-Y2 | 20 s | >99 | 10.7 | 1.13 | >0.95 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
|
(蔡中正, 刘野, 陶友华, 朱剑波, 化学学报, 2022, 80, 1165.)
doi: 10.6023/A22050235 |
|
| [9] |
|
| [10] |
|
| [11] |
|
|
(于慧萍, 秦亚伟, 董金勇, 化学进展, 2023, 35, 1294.)
doi: 10.7536/PC230229 |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
|
(李雅宁, 王晓艳, 唐勇, 化学学报, 2024, 82, 213.)
doi: 10.6023/A23100445 |
|
| [24] |
|
|
(王玉娜, 王超, 马海燕, 化学学报, 2025, 83, 25.)
doi: 10.6023/A24090275 |
|
| [25] |
doi: 10.1021/jacs.3c02348 pmid: 37171258 |
| [26] |
|
| [27] |
doi: 10.1126/science.adg4520 pmid: 37023198 |
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
|
(黄皓毅, 蔡中正, 朱剑波, 科学通报, 2023, 68, 4597.)
|
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
doi: 10.1016/j.tet.2008.03.108 pmid: 19606203 |
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
|
(廖曦, 秦娇娇, 唐小燕, 高分子学报, 2023, 54, 1426.)
|
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
pmid: 16898798 |
| [50] |
|
|
(蒋胜杰, 王杨, 徐信, 有机化学, 2023, 43, 1786.)
doi: 10.6023/cjoc202301026 |
|
| [51] |
|
|
(汪阳, 阎敬灵, 化学学报, 2023, 81, 275.)
doi: 10.6023/A23010004 |
|
| [52] |
|
|
(戈丁浩, 石枫, 有机化学, 2025, 45, 717.)
doi: 10.6023/cjoc202500004 |
|
| [53] |
pmid: 11841301 |
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [1] | 陶泽凡, 王椿焱, 杨大伟, 曲景平. 邻氨基苯硫酚桥联双金属配合物的合成、表征和反应性[J]. 化学学报, 2025, 83(7): 702-708. |
| [2] | 王玉娜, 王超, 马海燕. 苯并噁唑取代氨基酚氧基锌氯化物催化外消旋丙交酯开环聚合研究[J]. 化学学报, 2025, 83(1): 25-35. |
| [3] | 陈元金, 黄大江, 石向辉, 席振峰, 魏俊年. 还原条件下非对称钳形PNN钴配合物的反应性研究[J]. 化学学报, 2024, 82(5): 471-476. |
| [4] | 王镜焱, 马海燕. 2,6-二亚甲基吡啶桥联双(氨基酚氧基)钠、钾配合物的合成及催化外消旋丙交酯开环聚合研究[J]. 化学学报, 2024, 82(10): 1058-1068. |
| [5] | 汪阳, 阎敬灵. 不同配体的稀土金属配合物在烯烃聚合领域的研究进展[J]. 化学学报, 2023, 81(3): 275-288. |
| [6] | 杨贯文, 伍广朋. 模块化双功能有机硼氮和硼磷催化体系的设计及其催化转化★[J]. 化学学报, 2023, 81(11): 1551-1565. |
| [7] | 李波, 周海燕, 马海燕, 黄吉玲. 亚乙基桥联双茚锆、铪配合物的合成及催化丙烯选择性齐聚研究: 茚环3-位取代基的影响[J]. 化学学报, 2023, 81(10): 1280-1294. |
| [8] | 马雪璐, 李蒙, 雷鸣. 三核过渡金属配合物在催化反应中的研究进展[J]. 化学学报, 2023, 81(1): 84-99. |
| [9] | 朱诗敏, 黄鑫, 韩勰, 刘思敏. N^C^N型Pt(II)配合物与大环主体葫芦[10]脲的识别及发光性质研究[J]. 化学学报, 2022, 80(8): 1066-1070. |
| [10] | 江金辉, 朱云卿, 杜建忠. 开环聚合诱导自组装的挑战与展望[J]. 化学学报, 2020, 78(8): 719-724. |
| [11] | 李荣烨, Khiman Mehul, 盛力, 孙静. 两亲性聚氨基酸三嵌段共聚物构筑pH/溶剂可控多级纳米结构[J]. 化学学报, 2020, 78(11): 1235-1239. |
| [12] | 布美热木·克力木, 马海燕. 非对称β-二亚氨基镁络合物催化丙交酯、己内酯开环聚合/共聚研究[J]. 化学学报, 2018, 76(2): 121-132. |
| [13] | 崔彬彬, 唐健洪, 钟羽武. 基于过渡金属配合物的阻变存储材料[J]. 化学学报, 2016, 74(9): 726-733. |
| [14] | 张益伟, 马雪璐, 张欣, 雷鸣. 邻苯二硫酚桥联双核双氮过渡金属配合物N-N键活化规律的理论研究[J]. 化学学报, 2016, 74(4): 340-350. |
| [15] | 钱长涛, 王春红, 陈耀峰. 稀土金属有机配合物化学60年[J]. 化学学报, 2014, 72(8): 883-905. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||