化学学报 ›› 2025, Vol. 83 ›› Issue (8): 803-809.DOI: 10.6023/A25050151 上一篇 下一篇
研究通讯
投稿日期:2025-05-09
发布日期:2025-07-02
通讯作者:
李伟
作者简介:★ “中国青年化学家”专辑.
基金资助:
Weilong Zeng, Haosong Wang, Mingyang Wang, Wei Li*( )
)
Received:2025-05-09
Published:2025-07-02
Contact:
Wei Li
About author:★ For the VSI “Rising Stars in Chemistry”.
Supported by:文章分享
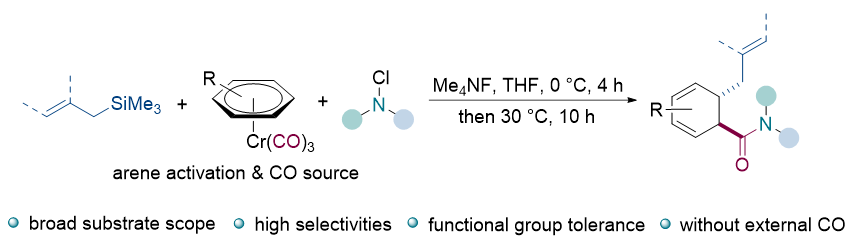
基于三羰基铬的双功能配位策略, 实现了简单芳烃的去芳构1,2-烯丙基化/氨羰基化反应. 该反应能够将重要的烯丙基和氨羰基官能团一步高选择性地整合到环状体系中, 从而快速制备了一系列含环己二烯环的β-烯丙基化酰胺化合物. 体系中, Cr(CO)3单元通过η6-配位模式不仅能够活化惰性苯环大π键促使其发生去芳构化反应, 同时也可以提供羰基化过程中所需的CO源. 去芳构羰基化方法无需使用外加有毒CO气体, 具备较好的底物普适性以及官能团兼容性, 特别是能适用于甲苯、苯甲醚、氟苯、氯苯等简单芳烃底物, 具有好的合成应用潜力.
曾伟龙, 王浩松, 汪名扬, 李伟. 铬介导芳烃的去芳构1,2-烯丙基化/氨羰基化反应★[J]. 化学学报, 2025, 83(8): 803-809.
Weilong Zeng, Haosong Wang, Mingyang Wang, Wei Li. Dearomative 1,2-Allylation/Aminocarbonylation Reaction of Chromium-Bound Arenes★[J]. Acta Chimica Sinica, 2025, 83(8): 803-809.
| Entry | Deviation from standard conditions | Yieldb/% |
|---|---|---|
| 1 | none | 82 |
| 2 | 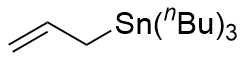 instead of instead of 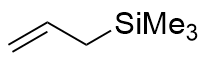 | 0 |
| 3 | KOtBu instead of Me4NF | 0 |
| 4 | TBAF instead of Me4NF | Trace |
| 5 | DCM instead of THF | 0 |
| 6 | MeCN instead of THF | 8 |
| 7 | DMSO instead of THF | 55 |
| 8 | DME instead of THF | 66 |
| 9 | EtOAc instead of THF | 71 |
| 10 | DMA instead of THF | 74 |
| 11 | DMF instead of THF | 81 |
| 12 | 30 ℃ instead of 0 ℃ | 77 |
| 13 | -10 ℃ instead of 0 ℃ | 71 |
| 14 | -45 ℃ instead of 0 ℃ | 79 |
| Entry | Deviation from standard conditions | Yieldb/% |
|---|---|---|
| 1 | none | 82 |
| 2 | 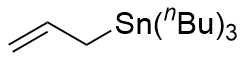 instead of instead of  | 0 |
| 3 | KOtBu instead of Me4NF | 0 |
| 4 | TBAF instead of Me4NF | Trace |
| 5 | DCM instead of THF | 0 |
| 6 | MeCN instead of THF | 8 |
| 7 | DMSO instead of THF | 55 |
| 8 | DME instead of THF | 66 |
| 9 | EtOAc instead of THF | 71 |
| 10 | DMA instead of THF | 74 |
| 11 | DMF instead of THF | 81 |
| 12 | 30 ℃ instead of 0 ℃ | 77 |
| 13 | -10 ℃ instead of 0 ℃ | 71 |
| 14 | -45 ℃ instead of 0 ℃ | 79 |
| [1] |
(a)
|
|
(b)
|
|
| [2] |
(a) The Chemistry of Alkenes, Ed.: Patai, S., John Wiley & Sons, London, 1964.
|
|
(b) Alkenes and Aromatics, Eds.: Taylor, P. G.; Gagan, J. M. F., RSC, Cambridge, 2002.
|
|
|
(c) Modern Arene Chemistry: Concepts, Synthesis, and Applications, Ed.: Astruc, D., Wiley-VCH, Weinheim, 2002.
|
|
|
(d) Arene Chemistry: Reaction Mechanisms and Methods for Aromatic Compounds, Ed.: Mortier, J., John Wiley & Sons, New Jersey, 2016.
|
|
| [3] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
|
(f)
|
|
|
(g)
|
|
|
(h)
|
|
|
(i)
|
|
|
(j)
|
|
|
(k)
|
|
|
(l)
|
|
|
(m)
|
|
|
(n)
|
|
| [4] |
For selected reviews on arene dearomatization, see: (a)
pmid: 11848895 |
|
(b)
pmid: 11848895 |
|
|
(c)
pmid: 11848895 |
|
|
(d)
pmid: 11848895 |
|
|
(e) Asymmetric Dearomatization Reactions, Ed.: You, S.-L., Wiley-VCH, 2016.
pmid: 11848895 |
|
|
(f)
pmid: 11848895 |
|
|
(g)
pmid: 11848895 |
|
|
(h)
pmid: 11848895 |
|
|
(i)
pmid: 11848895 |
|
|
(j)
pmid: 11848895 |
|
|
(朱敏, 张霄, 游书力, 高等学校化学学报, 2020, 41, 1407.)
doi: 10.7503/cjcu20200205 pmid: 11848895 |
|
|
(k)
pmid: 11848895 |
|
|
(程远征, 李木子, 王瑞祥, 祝龙浩, 沈文杰, 邹馨璇, 顾庆, 游书力, 化学进展, 2024, 36, 1785.)
doi: 10.7536/PC241203 pmid: 11848895 |
|
|
(l)
pmid: 11848895 |
|
|
(吴文挺, 张立明, 游书力, 化学学报, 2017, 75, 419.)
doi: 10.6023/A17020049 pmid: 11848895 |
|
| [5] |
For three-dimensional structures in medicinal chemistry, see: (a)
doi: 10.1021/jm901241e pmid: 24472495 |
|
(b)
pmid: 24472495 |
|
|
(c)
doi: 10.1021/jm101356p pmid: 24472495 |
|
|
(d)
pmid: 24472495 |
|
|
(e)
doi: 10.1111/cbdd.12260 pmid: 24472495 |
|
| [6] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
|
(f)
|
|
|
(g)
|
|
|
(i)
|
|
| [7] |
(a)
doi: 10.1021/cr9902852 pmid: 11749310 |
|
(b)
pmid: 11749310 |
|
|
(c)
pmid: 11749310 |
|
|
(d)
pmid: 11749310 |
|
|
(e)
pmid: 11749310 |
|
|
(高炜洋, 邓伟超, 高扬, 梁仁校, 贾义霞, 化学学报, 2024, 82, 1.)
doi: 10.6023/A23100472 pmid: 11749310 |
|
|
(f)
doi: 10.6023/A20110520 pmid: 11749310 |
|
|
(周波, 梁仁校, 曹中艳, 周平海, 贾义霞, 化学学报, 2021, 79, 176.)
doi: 10.6023/A20110520 pmid: 11749310 |
|
|
(g)
pmid: 11749310 |
|
| [8] |
For recent asymmetric reactions involving η6-coordination, see: (a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
| [9] |
For selected examples of chromium-catalyzed reactions, see: (a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
|
(袁芳艳, 李超, 罗美明, 曾小明, 化学学报, 2023, 81, 456.)
doi: 10.6023/A23020048 |
|
| [10] |
For selected reviews, see: (a)
pmid: 33739109 |
|
(b)
pmid: 33739109 |
|
|
(c)
pmid: 33739109 |
|
|
(d)
doi: 10.1021/acs.chemrev.7b00692 pmid: 33739109 |
|
|
(e)
pmid: 33739109 |
|
|
(f)
pmid: 33739109 |
|
|
(g)
doi: 10.1021/acs.chemrev.0c00736 pmid: 33739109 |
|
|
(h)
pmid: 33739109 |
|
|
(i)
pmid: 33739109 |
|
|
(j)
pmid: 33739109 |
|
|
(k)
pmid: 33739109 |
|
|
(l)
pmid: 33739109 |
|
|
(m)
pmid: 33739109 |
|
|
(汤淏溟, 霍小红, 孟庆华, 张万斌, 化学学报, 2016, 74, 219.)
doi: 10.6023/A16020078 pmid: 33739109 |
|
| [11] |
For selected examples, see: (a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
|
(f)
|
|
|
(g)
doi: 10.1039/c9qo01049a |
|
|
(h)
|
|
|
(i)
doi: 10.1016/S1872-2067(23)64461-4 |
|
|
(j)
|
|
|
(米一曼, 黄学良, 有机化学, 2023, 43, 359.)
doi: 10.6023/cjoc202300002 |
|
| [12] |
(a)
pmid: 26571338 |
|
(b)
pmid: 26571338 |
|
|
(c)
pmid: 26571338 |
|
|
(d)
doi: 10.1021/acs.jmedchem.5b01409 pmid: 26571338 |
|
|
(e)
pmid: 26571338 |
|
|
(f)
pmid: 26571338 |
|
|
(李龙, 周波, 叶龙武, 有机化学, 2015, 35, 655.)
doi: 10.6023/cjoc201412009 pmid: 26571338 |
|
|
(g)
pmid: 26571338 |
|
|
(李远明, 贾凡, 马丽娜, 李志平, 化学学报, 2015, 73, 1311.)
doi: 10.6023/A15040287 pmid: 26571338 |
|
|
(h)
pmid: 26571338 |
|
|
(黄培强, 化学学报, 2018, 76, 357.)
doi: 10.6023/A18020054 pmid: 26571338 |
|
|
(i)
pmid: 26571338 |
|
|
(李春, 王梦娜, 陆逊花, 杨元勇, 张林, 有机化学, 2019, 39, 1109.)
doi: 10.6023/cjoc201809020 pmid: 26571338 |
|
|
(j)
pmid: 26571338 |
|
|
(李国凯, 朱滨锋, 胡涛, 樊瑞峰, 孙蔚青, 和振秀, 陈景超, 樊保敏, 化学学报, 2025, 83, 199.)
doi: 10.6023/A24100307 pmid: 26571338 |
| [1] | 张大伟, 赵海洋, 冯笑甜, 顾玉诚, 张新刚. 钯催化下杂芳基溴代物与偕二氟烯丙基硼试剂的交叉偶联反应[J]. 化学学报, 2024, 82(2): 105-109. |
| [2] | 许木榕, 周纯, 王子慧, 杨丽冰, 李晨远, 卓炜丰, 王行之, 杜克钊. 固态溴素合成溴化物晶体的研究——以CrSBr合成和表征为例[J]. 化学学报, 2024, 82(12): 1234-1240. |
| [3] | 李晖, 殷亮. 一价铜催化的酮或酮亚胺的不对称烯丙基化反应研究进展[J]. 化学学报, 2024, 82(12): 1274-1288. |
| [4] | 高炜洋, 邓伟超, 高扬, 梁仁校, 贾义霞. 吲哚分子间不对称去芳构化氧化Heck反应[J]. 化学学报, 2024, 82(1): 1-4. |
| [5] | 袁芳艳, 李超, 罗美明, 曾小明. 铬催化酮羰基的脱氧偶联反应合成四取代烯烃★[J]. 化学学报, 2023, 81(5): 456-460. |
| [6] | 张瑾, 丁湘浓, 刘红超, 樊栋, 徐舒涛, 魏迎旭, 刘中民. HMOR分子筛骨架铝分布研究及二甲醚羰基化反应活性中心的辨识※[J]. 化学学报, 2022, 80(5): 590-597. |
| [7] | 张丹琪, 邵英博, 郑汉良, 周碧莹, 薛小松. 双齿氮配体螯合五价碘试剂介导的苯酚氧化去芳构化机理的理论研究[J]. 化学学报, 2021, 79(11): 1394-1400. |
| [8] | 董金龙, 沈腊珍, 文斌, 宋珍, 冯俊杰, 梁钢, 刘斌, 杨斌盛. 新型抗糖尿病铬(III)配合物的合成和生物活性及机理探究[J]. 化学学报, 2020, 78(11): 1260-1267. |
| [9] | 王明, 姜雪峰. 二芳基碘嗡盐参与的CO/I交换策略构建9-芴酮[J]. 化学学报, 2018, 76(5): 377-381. |
| [10] | 周锵, 陆平. 手性路易斯碱和过渡金属协同催化反应的进展[J]. 化学学报, 2018, 76(11): 825-830. |
| [11] | 张金龙, 蒋高喜. 不对称烯丙基化反应合成含有三苯胺核心单元的荧光非天然氨基酸衍生物[J]. 化学学报, 2018, 76(11): 890-894. |
| [12] | 吴文挺, 张立明, 游书力. 金催化去芳构化反应研究进展[J]. 化学学报, 2017, 75(5): 419-438. |
| [13] | 张子競, 陶忠林, 阿拉法特·阿地力, 龚流柱. 钯配合物和手性磷酸连续催化的烯丙醇和醛的不对称羰基烯丙基化反应[J]. 化学学报, 2017, 75(12): 1196-1201. |
| [14] | 段德河, 殷勤, 王守国, 顾庆, 游书力. 手性磷酸催化的C(3)-取代吲哚和甲基乙烯基酮不对称串联反应[J]. 化学学报, 2014, 72(9): 1001-1004. |
| [15] | 王都留, 杨建东, 杨升宏, 郭锦秀. FePO4包覆Fe3O4磁性纳米微粒的合成及其在Cr(III)富集分离中的应用[J]. 化学学报, 2013, 71(9): 1287-1292. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||