化学学报 ›› 2025, Vol. 83 ›› Issue (12): 1538-1550.DOI: 10.6023/A25070259 上一篇 下一篇
研究论文
戴磊a, 葛明成a, 钟野a, 梁琳娜b, 蒋海燕c, 张建国a, 李志敏a,*( )
)
投稿日期:2025-07-18
发布日期:2025-09-11
基金资助:
Lei Daia, Mingcheng Gea, Ye Zhonga, Linna Liangb, Haiyan Jiangc, Jianguo Zhanga, Zhimin Lia,*( )
)
Received:2025-07-18
Published:2025-09-11
Contact:
* E-mail: lizm@bit.edu.cn
Supported by:文章分享
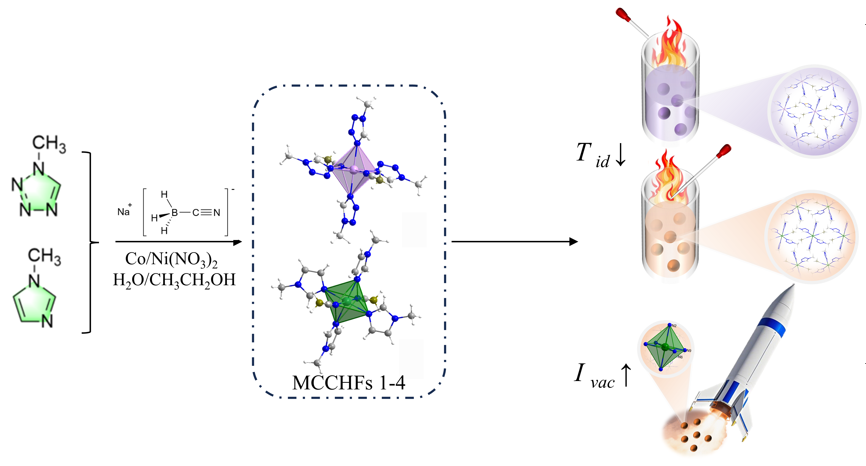
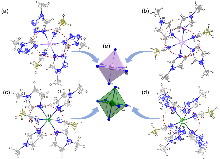
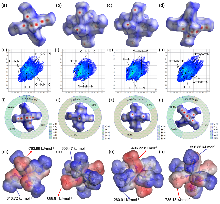
固液混合自燃推进剂在空间动力系统中有良好的应用前景, 为探索新型自燃固体燃料, 以Co(II)、Ni(II)为中心金属离子, 1-甲基咪唑、1-甲基四唑为配体, 氰基硼氢根为阴离子合成了4种金属配合物自燃燃料MCCHFs 1~4, 准确测定出其晶体结构, 并对它们的热行为、机械感度、自燃点火燃烧和能量性能进行了详细研究. 结果表明: 4种配合物的热分解峰温在152.4~176.3 ℃之间, MCCHF-1和MCCHF-4表现出一定的机械刺激敏感性(IS: 8 J, 17 J; FS: 288 N, 216 N), MCCHF-2和MCCHF-3对机械刺激钝感(IS>40 J, FS>360 N); 4种配合物均能与白烟硝酸发生自燃点火反应, 点火延迟时间处于2~57 ms范围, 其中MCCHF-1表现最佳, 仅为2 ms; 燃烧热值以MCCHF-2最高, 质量能量密度为25.41 kJ•g−1, 体积能量密度为32.17 kJ•cm−3, MCCHF-1、MCCHF-4与HNO3、N2O4、H2O2三种液体氧化剂组合, 表现出良好的比冲性能, 其中与H2O2组合的推进剂性能最佳, 真空比冲高达268.37 s, 高于偏二甲肼(258 s)等传统自燃燃料. 氰基硼氢阴离子的高活性金属配合物自燃燃料作为一种新型自燃固体燃料, 有望推动固液推进剂技术发展.
戴磊, 葛明成, 钟野, 梁琳娜, 蒋海燕, 张建国, 李志敏. 氰基硼氢金属配合物自燃燃料设计制备与性能研究[J]. 化学学报, 2025, 83(12): 1538-1550.
Lei Dai, Mingcheng Ge, Ye Zhong, Linna Liang, Haiyan Jiang, Jianguo Zhang, Zhimin Li. Design, Preparation and Performance of Cyanoborohydride Based Coordination Compounds for Hypergolic Fuels[J]. Acta Chimica Sinica, 2025, 83(12): 1538-1550.

| Item No. | MCCHF-1 | MCCHF-2 | MCCHF-3 | MCCHF-4 |
|---|---|---|---|---|
| compounds | Co(1-MTZ)4(CBH)2 | Co(MIM)4(CBH)2 | Ni(MIM)4(CBH)2 | Ni(1-MTZ)4(CBH)2 |
| formula | C10H22B2N18Co | C18H30B2N10Co | C18H30B2N10Ni | C10H22B2N18Ni |
| form. mass | 475.01 | 467.07 | 466.85 | 474.79 |
| T/K | 296.15 | 293(2) | 293(2) | 296(2) |
| crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| space group | P21/c | P21/n | P21/c | P21/c |
| a/nm | 0.74388(4) | 0.78597(7) | 0.78521(7) | 0.7377 |
| b/nm | 1.69365(10) | 1.10832(11) | 1.10513(11) | 1.6947 |
| c/nm | 0.94913(5) | 1.41131(13) | 1.56381(14) | 0.9412 |
| α/(°) | 90.00 | 90 | 90 | 90.00 |
| β/(°) | 108.7460(10) | 94.484(2) | 115.738(5) | 108.58 |
| γ/(°) | 90.00 | 90 | 90 | 90.00 |
| V/nm3 | 1.13235(11) | 1.2256(2) | 1.2224(2) | 1.1154 |
| Z | 2 | 2 | 2 | 2 |
| ρcalcd/(g•cm−3) | 1.393 | 1.266 | 1.268 | 1.414 |
| μ/mm−1 | 0.796 | 0.725 | 0.819 | 0.908 |
| F(000) | 490 | 490 | 492 | 492 |
| Goodness-of-fit on F2 | 1.050 | 1.034 | 1.042 | 1.165 |
| Final R indexes [I≥2σ(I)] | R1=0.0413, wR2=0.0842 | R1=0.0954, wR2=0.1554 | R1=0.0895, wR2=0.1861 | R1=0.0732, wR2=0.1662 |
| Final R indexes [all data] | R1=0.0640, wR2=0.0952 | R1=0.2156, wR2=0.1814 | R1=0.1928, wR2=0.2109 | R1=0.1196, wR2=0.1828 |
| CCDC | 2240151 | 2451525 | 2451527 | 2240152 |
| Item No. | MCCHF-1 | MCCHF-2 | MCCHF-3 | MCCHF-4 |
|---|---|---|---|---|
| compounds | Co(1-MTZ)4(CBH)2 | Co(MIM)4(CBH)2 | Ni(MIM)4(CBH)2 | Ni(1-MTZ)4(CBH)2 |
| formula | C10H22B2N18Co | C18H30B2N10Co | C18H30B2N10Ni | C10H22B2N18Ni |
| form. mass | 475.01 | 467.07 | 466.85 | 474.79 |
| T/K | 296.15 | 293(2) | 293(2) | 296(2) |
| crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| space group | P21/c | P21/n | P21/c | P21/c |
| a/nm | 0.74388(4) | 0.78597(7) | 0.78521(7) | 0.7377 |
| b/nm | 1.69365(10) | 1.10832(11) | 1.10513(11) | 1.6947 |
| c/nm | 0.94913(5) | 1.41131(13) | 1.56381(14) | 0.9412 |
| α/(°) | 90.00 | 90 | 90 | 90.00 |
| β/(°) | 108.7460(10) | 94.484(2) | 115.738(5) | 108.58 |
| γ/(°) | 90.00 | 90 | 90 | 90.00 |
| V/nm3 | 1.13235(11) | 1.2256(2) | 1.2224(2) | 1.1154 |
| Z | 2 | 2 | 2 | 2 |
| ρcalcd/(g•cm−3) | 1.393 | 1.266 | 1.268 | 1.414 |
| μ/mm−1 | 0.796 | 0.725 | 0.819 | 0.908 |
| F(000) | 490 | 490 | 492 | 492 |
| Goodness-of-fit on F2 | 1.050 | 1.034 | 1.042 | 1.165 |
| Final R indexes [I≥2σ(I)] | R1=0.0413, wR2=0.0842 | R1=0.0954, wR2=0.1554 | R1=0.0895, wR2=0.1861 | R1=0.0732, wR2=0.1662 |
| Final R indexes [all data] | R1=0.0640, wR2=0.0952 | R1=0.2156, wR2=0.1814 | R1=0.1928, wR2=0.2109 | R1=0.1196, wR2=0.1828 |
| CCDC | 2240151 | 2451525 | 2451527 | 2240152 |

| MCCHF-1 | MCCHF-2 | MCCHF-3 | MCCHF-4 | |
|---|---|---|---|---|
| Ma/(g•mol−1) | 475.01 | 467.07 | 466.85 | 474.79 |
| ρb/(g•cm−3) | 1.393 | 1.266 | 1.268 | 1.414 |
| tidc/ms | 2 | 20 | 57 | 37 |
| Tp d/℃ | 152.4 | 176.3 | 171.8 | 168.8 |
| Eg e/(kJ•g−1) | 20.44 | 25.41 | 23.77 | 18.36 |
| Ev f/(kJ•cm−3) | 28.47 | 32.17 | 30.14 | 25.97 |
| ISg/J | 8 | >40 | >40 | 17 |
| FSh/N | 288 | >360 | >360 | 216 |
| ΔUc i/(J•g−1) | -20433.05 | -25434.78 | -23746.10 | -18367.83 |
| ΔHc j/(kJ•mol−1) | -9709.2 | -11868.24 | -11097.02 | -8717.14 |
| ΔHf k/(kJ•mol−1) | 1051.95 | -1080.41 | -1786.25 | 125.27 |
| MCCHF-1 | MCCHF-2 | MCCHF-3 | MCCHF-4 | |
|---|---|---|---|---|
| Ma/(g•mol−1) | 475.01 | 467.07 | 466.85 | 474.79 |
| ρb/(g•cm−3) | 1.393 | 1.266 | 1.268 | 1.414 |
| tidc/ms | 2 | 20 | 57 | 37 |
| Tp d/℃ | 152.4 | 176.3 | 171.8 | 168.8 |
| Eg e/(kJ•g−1) | 20.44 | 25.41 | 23.77 | 18.36 |
| Ev f/(kJ•cm−3) | 28.47 | 32.17 | 30.14 | 25.97 |
| ISg/J | 8 | >40 | >40 | 17 |
| FSh/N | 288 | >360 | >360 | 216 |
| ΔUc i/(J•g−1) | -20433.05 | -25434.78 | -23746.10 | -18367.83 |
| ΔHc j/(kJ•mol−1) | -9709.2 | -11868.24 | -11097.02 | -8717.14 |
| ΔHf k/(kJ•mol−1) | 1051.95 | -1080.41 | -1786.25 | 125.27 |
| 参数 | MCCHF-1 | MCCHF-2 | MCCHF-3 | MCCHF-4 | |
|---|---|---|---|---|---|
| β/(℃•min−1) | 5 | 173.5 | 200.2 | 209.6 | 172.8 |
| 10 | 177.7 | 203.5 | 213.3 | 177.6 | |
| 15 | 180.5 | 206.4 | 216.2 | 179.8 | |
| 20 | 182.4 | 209.5 | 218.5 | 181.7 | |
| Kissinger法 | EK/(kJ•mol−1) | 255.3 | 274.7 | 299.3 | 256.3 |
| lg AK | 27.97 | 28.44 | 30.51 | 28.21 | |
| RK | -0.9996 | -0.9836 | -0.9957 | -0.9986 | |
| Ozawa法 | EO/(kJ•mol−1) | 249.9 | 268.8 | 292.3 | 250.8 |
| RO | -0.9997 | -0.9845 | -0.9959 | -0.9986 | |
| Ea/(kJ•mol−1) | 256.2 | 271.7 | 295.8 | 253.5 | |
| Tpo a/℃ | 167.40 | 195.90 | 204.90 | 163.10 | |
| Tb b/℃ | 173.91 | 202.75 | 211.42 | 169.45 | |
| ΔS≠ c/(J•K−1•mol−1) | 287.29 | 295.77 | 335.24 | 291.97 | |
| ΔH≠ d/(kJ•mol−1) | 251.64 | 270.80 | 295.33 | 252.67 | |
| ΔG≠ e/(kJ•mol−1) | 126.07 | 132.07 | 135.06 | 125.30 | |
| 参数 | MCCHF-1 | MCCHF-2 | MCCHF-3 | MCCHF-4 | |
|---|---|---|---|---|---|
| β/(℃•min−1) | 5 | 173.5 | 200.2 | 209.6 | 172.8 |
| 10 | 177.7 | 203.5 | 213.3 | 177.6 | |
| 15 | 180.5 | 206.4 | 216.2 | 179.8 | |
| 20 | 182.4 | 209.5 | 218.5 | 181.7 | |
| Kissinger法 | EK/(kJ•mol−1) | 255.3 | 274.7 | 299.3 | 256.3 |
| lg AK | 27.97 | 28.44 | 30.51 | 28.21 | |
| RK | -0.9996 | -0.9836 | -0.9957 | -0.9986 | |
| Ozawa法 | EO/(kJ•mol−1) | 249.9 | 268.8 | 292.3 | 250.8 |
| RO | -0.9997 | -0.9845 | -0.9959 | -0.9986 | |
| Ea/(kJ•mol−1) | 256.2 | 271.7 | 295.8 | 253.5 | |
| Tpo a/℃ | 167.40 | 195.90 | 204.90 | 163.10 | |
| Tb b/℃ | 173.91 | 202.75 | 211.42 | 169.45 | |
| ΔS≠ c/(J•K−1•mol−1) | 287.29 | 295.77 | 335.24 | 291.97 | |
| ΔH≠ d/(kJ•mol−1) | 251.64 | 270.80 | 295.33 | 252.67 | |
| ΔG≠ e/(kJ•mol−1) | 126.07 | 132.07 | 135.06 | 125.30 | |

| 输入参数 | 来源 | |
|---|---|---|
| 燃料 | MCCHF-1 (C10H22B2N18Co) MCCHF-4 (C10H22B2N18Ni) | 实验室自制 |
| 氧化剂 | HNO3/H2O2/N2O4 | NASA-CEA默认数据库 |
| 氧燃比 | 1.0~10.0 | 参数化研究 |
| 生成焓(ΔHf) | MCCHF-1: 1051.95 kJ•mol−1 MCCHF-4: 125.27 kJ•mol−1 | 氧弹量热仪实测 |
| 燃烧室压力(Pc) | 2.533 MPa | 文献[51] |
| 喷管出口压力(Pe) | 0.1013 MPa | |
| 喷管面积比(Ae/At) | 4 | |
| 流动模型 | IAC模型 | |
| 输入参数 | 来源 | |
|---|---|---|
| 燃料 | MCCHF-1 (C10H22B2N18Co) MCCHF-4 (C10H22B2N18Ni) | 实验室自制 |
| 氧化剂 | HNO3/H2O2/N2O4 | NASA-CEA默认数据库 |
| 氧燃比 | 1.0~10.0 | 参数化研究 |
| 生成焓(ΔHf) | MCCHF-1: 1051.95 kJ•mol−1 MCCHF-4: 125.27 kJ•mol−1 | 氧弹量热仪实测 |
| 燃烧室压力(Pc) | 2.533 MPa | 文献[51] |
| 喷管出口压力(Pe) | 0.1013 MPa | |
| 喷管面积比(Ae/At) | 4 | |
| 流动模型 | IAC模型 | |
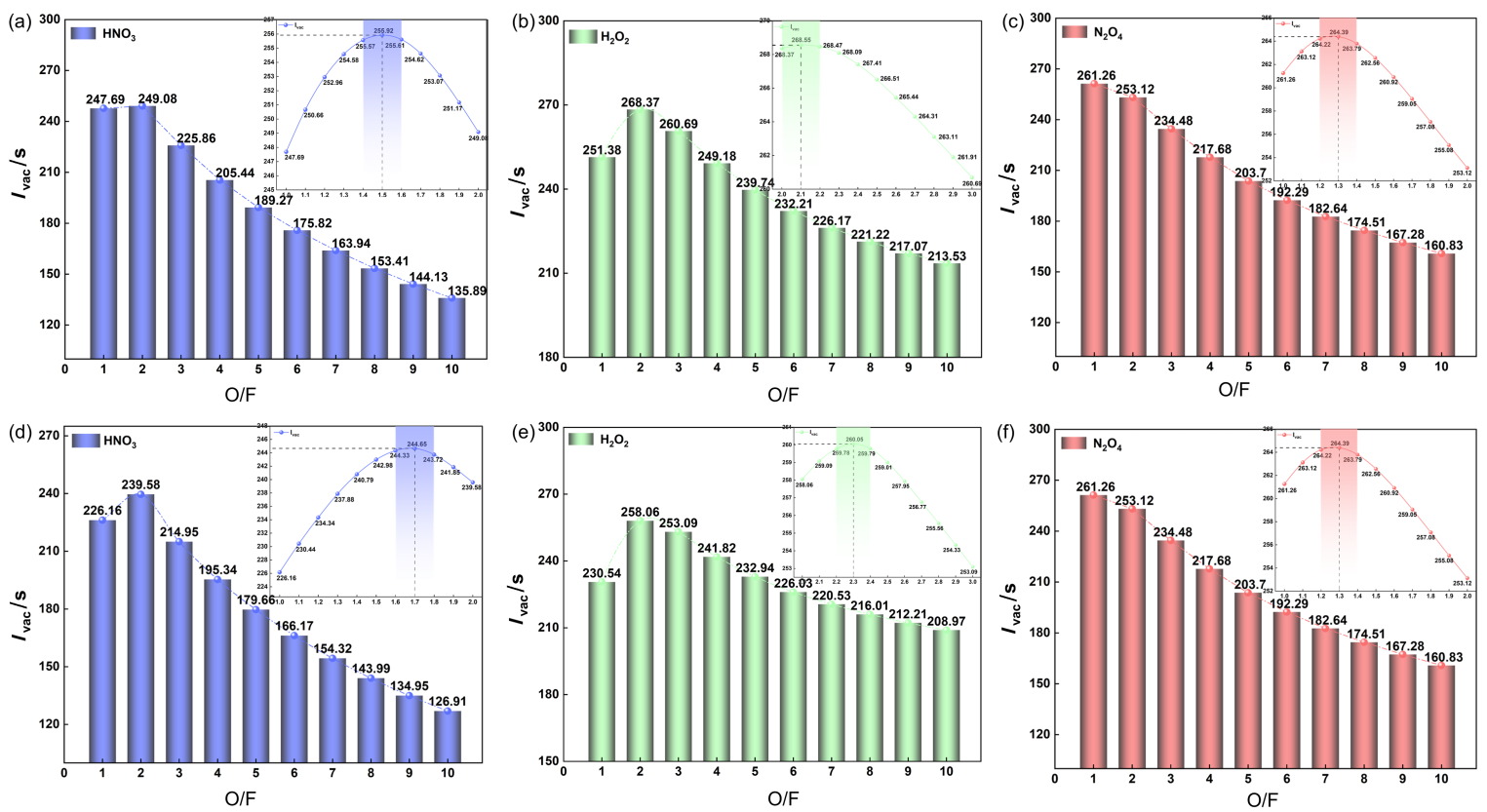
| Hypergolic-fuels | MCCHF-1 | MCCHF-4 | MMH[ | UDMH[ | |||||
|---|---|---|---|---|---|---|---|---|---|
| HNO3 | N2O4 | H2O2 | HNO3 | N2O4 | H2O2 | ||||
| O/F | 1.5 | 1.3 | 2.1 | 1.7 | 1.4 | 2.3 | 2.5 | 3 | |
| Ivac/s | 255.92 | 264.43 | 268.57 | 244.65 | 252.77 | 260.05 | 260 | 258 | |
| Isp/s | 225.76 | 237.73 | 236.07 | 215.44 | 222.48 | 229.11 | — — | ||
| 燃烧室温度/K | 3079.93 | 3338.88 | 2963.39 | 2933.77 | 3162.48 | 2842.46 | |||
| 特征速度/(m•s−1) | 1544.4 | 1595.4 | 1619.2 | 1474.3 | 1522.5 | 1567.8 | |||
| 燃烧产物的摩尔质量/(kg•kmol−1) | 26.60 | 26.86 | 23.38 | 27.89 | 27.96 | 23.92 | |||
| Hypergolic-fuels | MCCHF-1 | MCCHF-4 | MMH[ | UDMH[ | |||||
|---|---|---|---|---|---|---|---|---|---|
| HNO3 | N2O4 | H2O2 | HNO3 | N2O4 | H2O2 | ||||
| O/F | 1.5 | 1.3 | 2.1 | 1.7 | 1.4 | 2.3 | 2.5 | 3 | |
| Ivac/s | 255.92 | 264.43 | 268.57 | 244.65 | 252.77 | 260.05 | 260 | 258 | |
| Isp/s | 225.76 | 237.73 | 236.07 | 215.44 | 222.48 | 229.11 | — — | ||
| 燃烧室温度/K | 3079.93 | 3338.88 | 2963.39 | 2933.77 | 3162.48 | 2842.46 | |||
| 特征速度/(m•s−1) | 1544.4 | 1595.4 | 1619.2 | 1474.3 | 1522.5 | 1567.8 | |||
| 燃烧产物的摩尔质量/(kg•kmol−1) | 26.60 | 26.86 | 23.38 | 27.89 | 27.96 | 23.92 | |||
| [1] |
doi: 10.1016/j.combustflame.2017.03.021 |
| [2] |
|
| [3] |
doi: 10.6023/A24010017 |
|
(崔勇康, 成守飞, 凌琳, 李玉学, 吕龙, 化学学报, 2024, 82, 377.)
doi: 10.6023/A24010017 |
|
| [4] |
doi: 10.3390/en18020267 |
| [5] |
doi: 10.1016/j.ijhydene.2025.01.403 |
| [6] |
|
| [7] |
doi: 10.1002/prep.v45.11 |
| [51] |
doi: 10.1016/j.combustflame.2020.01.013 |
| [52] |
doi: 10.1021/ac60131a045 |
| [53] |
doi: 10.1246/bcsj.38.1881 |
| [54] |
doi: 10.1107/S1600576721002910 |
| [55] |
doi: 10.1002/prep.v40.1 |
| [56] |
doi: 10.1002/anie.v53.11 |
| [57] |
|
| [8] |
doi: 10.1016/j.tca.2023.179562 |
| [9] |
|
|
(蔡国飙, 推进技术, 2012, 6, 831.)
|
|
| [10] |
doi: 10.1016/j.ast.2006.08.008 |
| [11] |
doi: 10.1016/j.fpc.2021.11.010 |
| [12] |
|
|
(陈启航, 付小龙, 蔚红建, 孟赛钦, 兵器装备工程学报, 2025, 46, 279.)
|
|
| [13] |
doi: 10.1016/j.fuel.2019.115729 |
| [14] |
doi: 10.1016/j.combustflame.2019.10.029 |
| [15] |
|
|
(王印, 胡松启, 刘林林, 李连强, 推进技术, 2020, 48, 1807.)
|
|
| [16] |
|
|
(张泽林, 林鑫, 王若岩, 罗家枭, 王泽众, 张春元, 李飞, 余西龙, 推进技术, 2023, 44, 2211059.)
|
|
| [17] |
|
|
(杨玉新, 胡春波, 何国强, 蔡体敏, 宇航学报, 2008, 5, 1616.)
|
|
| [18] |
|
| [19] |
|
| [20] |
doi: 10.3866/PKU.WHXB201812031 |
|
(郑东, 熊鹏飞, 钟北京, 物理化学学报, 2019, 35, 1241.)
|
|
| [21] |
doi: 10.1016/j.cej.2019.122623 |
| [22] |
|
|
(林彩霞, 郭琳, 李庆山, 张正之, 袁耀锋, 徐凤波, 有机化学, 2014, 34, 239.)
doi: 10.6023/cjoc201307055 |
|
| [23] |
doi: 10.1002/adma.v28.28 |
| [24] |
doi: 10.6023/A23020056 |
|
(杨洁, 凌琳, 李玉学, 吕龙, 化学学报, 2023, 81, 328.)
doi: 10.6023/A23020056 |
|
| [25] |
doi: 10.6023/A19120419 |
|
(武艳, 庞爱民, 胡磊, 何根升, 张莹莹, 张利雄, 李明海, 马振叶, 化学学报, 2020, 78, 337.)
doi: 10.6023/A19120419 |
|
| [26] |
doi: 10.6023/A25040108 |
|
(喻尧, 李晓强, 刘佳鑫, 顾军, 涂涛, 杨军, 化学学报, 2025, 83, 1018.)
doi: 10.6023/A25040108 |
|
| [27] |
doi: 10.6023/A22100425 |
|
(马雪璐, 李蒙, 雷鸣, 化学学报, 2023, 81, 84.)
doi: 10.6023/A22100425 |
|
| [28] |
doi: 10.3390/ma16010067 |
| [29] |
|
|
(杨利, 张国英, 刘影, 张同来, 物理化学学报, 2017, 33, 2463.)
|
|
| [30] |
doi: 10.1039/c8qi01279b |
| [31] |
doi: 10.1039/C8NJ04143A |
| [32] |
doi: 10.1016/j.cej.2024.157616 |
| [33] |
|
| [34] |
doi: 10.1016/j.fuel.2024.132615 |
| [35] |
doi: 10.1016/j.combustflame.2021.111556 |
| [36] |
doi: 10.1016/j.combustflame.2020.10.035 |
| [37] |
doi: 10.1021/acs.inorgchem.2c02479 |
| [38] |
|
| [39] |
doi: 10.1016/j.fuel.2025.134683 |
| [40] |
doi: 10.1039/D2QI00968D |
| [41] |
doi: 10.1039/C6TA02699K |
| [42] |
doi: 10.1080/07370652.2020.1770897 |
| [43] |
|
| [44] |
doi: 10.1021/acs.inorgchem.1c00109 |
| [45] |
doi: 10.1021/acs.cgd.4c01648 |
| [46] |
|
|
(费腾, 徐冉, 赵鹏宇, 徐涛, 杜宗罡, 火箭推进, 2024, 50, 33.)
|
|
| [47] |
|
|
(武颖韬, 费立涵, 孔祥东, 王帜, 汤成龙, 黄佐华, 化工学报, 2024, 75, 2017.)
doi: 10.11949/0438-1157.20240102 |
|
| [48] |
doi: 10.1016/j.cej.2021.131866 |
| [49] |
|
| [50] |
doi: 10.1016/j.jcis.2007.04.028 |
| [1] | 喻尧, 李晓强, 刘佳鑫, 顾军, 涂涛, 杨军. 端羟基聚丁二烯(HTPB)接枝含硅双核二茂铁衍生物的合成及其应用性能研究[J]. 化学学报, 2025, 83(9): 1018-1024. |
| [2] | 崔勇康, 成守飞, 凌琳, 李玉学, 吕龙. 二氟氨基二硝甲基芳香杂环含能材料的理论研究[J]. 化学学报, 2024, 82(4): 377-386. |
| [3] | 杨洁, 凌琳, 李玉学, 吕龙. 高氯酸铵热分解机理的密度泛函理论研究[J]. 化学学报, 2023, 81(4): 328-337. |
| [4] | 吕少一, 邵自强, 张振玲, 王慧庆, 王文俊. 新型含能纤维素基凝胶推进剂的流变性能研究[J]. 化学学报, 2012, 70(02): 200-206. |
| [5] | 胡磊, 马振叶, 纪明卫, 张利雄. 纳米Fe2O3/端羟基聚丁二烯(HTPB)复合粒子的制备与表征[J]. 化学学报, 2011, 69(24): 3028-3032. |
| [6] | 冯金玲, 张建国, 李志敏, 张同来, 崔燕, 杨利. 高氮含能配合物[Co(AZT)2(H2O)4](HTNR)2•4H2O的合成、晶体结构及性质[J]. 化学学报, 2010, 68(24): 2493-2499. |
| [7] | 付一政, 胡双启, 兰艳花, 刘亚青. HTPB/增塑剂玻璃化转变温度及力学性能的分子动力学模拟[J]. 化学学报, 2010, 68(08): 809-813. |
| [8] | 尚静, 张建国, 崔燕, 张同来, 舒远杰, 杨利. 含能配合物[Zn(DAT)6](ClO4)2(DAT=1,5-二氨基四唑)的合成、晶体结构及性质[J]. 化学学报, 2010, 68(03): 233-238. |
| [9] | 周春生a,b 范 广a 陈三平a 赵凤起c 焦宝娟a,d 高胜利,a. 含能配合物[Mn(BTA)(phen)2•5H2O]n的合成、结构与性质研究[J]. 化学学报, 2008, 66(15): 1776-1780. |
| [10] | 陈沛, 赵凤起, 罗阳, 胡荣祖, 郑玉梅, 邓敏智, 高茵. 2-羟基和4-羟基-3,5-二硝基吡啶铅盐的热行为、分解机理、非等温分解反应动力学及其在推进剂中的应用[J]. 化学学报, 2004, 62(13): 1197-1204. |
| [11] | 洪品杰,伍宗敏. 按非刚性转子模型计算NF3和环氧乙烷的光谱熵[J]. 化学学报, 1983, 41(11): 1065-1066. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||