化学学报 ›› 2025, Vol. 83 ›› Issue (12): 1465-1471.DOI: 10.6023/A25070265 上一篇 下一篇
研究通讯
朱子琦a,*,†( ), 叶乐华a,†, 吴怡a,†, 郭梓圻a, 石枫a,b,*(
), 叶乐华a,†, 吴怡a,†, 郭梓圻a, 石枫a,b,*( )
)
投稿日期:2025-07-30
发布日期:2025-10-10
基金资助:
Ziqi Zhua,*( ), Lehua Yea, Yi Wua, Ziqi Guoa, Feng Shia,b,*(
), Lehua Yea, Yi Wua, Ziqi Guoa, Feng Shia,b,*( )
)
Received:2025-07-30
Published:2025-10-10
Contact:
* E-mail: zzq@cczu.edu.cn;fshi@jsnu.edu.cn
About author:Supported by:文章分享
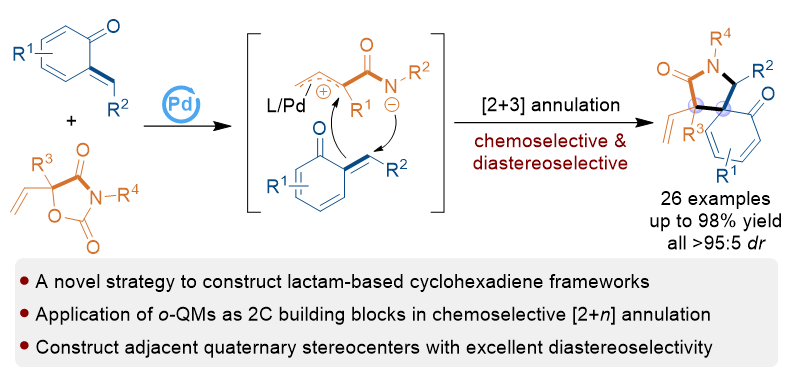
五元内酰胺螺环己二烯属于一类重要的螺环骨架, 但是该类骨架的构建策略非常有限和具有挑战性. 为了解决该类骨架构建中的挑战性问题, 本工作发展了钯催化下邻亚甲基苯醌与乙烯基𫫇唑二酮的化学选择性[2+3]环化反应, 以中等至高的产率(49%~98%)、优秀的非对映选择性(均>95∶5 dr)构建了具有连续季碳中心的五元内酰胺螺环己二烯骨架. 该转化不仅拓展了邻亚甲基苯醌作为二元合成砌块在环化反应中的应用, 而且为五元内酰胺螺环己二烯骨架的高效、高非对映选择性构建提供了新的策略. 此外, 对该[2+3]环化反应进行了初步的催化不对称研究, 显示钯-手性膦配体催化体系可以实现一定程度的对映选择性控制.
朱子琦, 叶乐华, 吴怡, 郭梓圻, 石枫. 邻亚甲基苯醌参与的化学选择性和非对映选择性[2+3]环化反应[J]. 化学学报, 2025, 83(12): 1465-1471.
Ziqi Zhu, Lehua Ye, Yi Wu, Ziqi Guo, Feng Shi. o-Quinone Methide-Involved Chemoselective and Diastereoselective [2+3] Annulation[J]. Acta Chimica Sinica, 2025, 83(12): 1465-1471.
| Entry | Metal | Ligand | Solvent | Yield/% | dr |
|---|---|---|---|---|---|
| 1 | Pd2(dba)3 | PPh3 | MeCN | 84 | >95∶5 |
| 2 | Pd(PPh3)2Cl2 | PPh3 | MeCN | 12 | >95∶5 |
| 3 | [Ir(cod)Cl]2 | PPh3 | MeCN | N.R. | — |
| 4 | CuOTf | PPh3 | MeCN | N.R. | — |
| 5 | Pd2(dba)3 | dppp | MeCN | 40 | >95∶5 |
| 6 | Pd2(dba)3 | dppf | MeCN | 36 | >95∶5 |
| 7 | Pd2(dba)3 | PPh3 | DCE | 95 | >95∶5 |
| 8 | Pd2(dba)3 | PPh3 | EtOAc | 83 | >95∶5 |
| 9 | Pd2(dba)3 | PPh3 | DMF | 62 | >95∶5 |
| 10 | Pd2(dba)3 | PPh3 | Toluene | 82 | >95∶5 |
| 11 | Pd2(dba)3 | PPh3 | THF | 76 | >95∶5 |
| 12b | Pd2(dba)3 | PPh3 | DCE | 86 | >95∶5 |
| 13c | Pd2(dba)3 | PPh3 | DCE | 68 | >95∶5 |
| Entry | Metal | Ligand | Solvent | Yield/% | dr |
|---|---|---|---|---|---|
| 1 | Pd2(dba)3 | PPh3 | MeCN | 84 | >95∶5 |
| 2 | Pd(PPh3)2Cl2 | PPh3 | MeCN | 12 | >95∶5 |
| 3 | [Ir(cod)Cl]2 | PPh3 | MeCN | N.R. | — |
| 4 | CuOTf | PPh3 | MeCN | N.R. | — |
| 5 | Pd2(dba)3 | dppp | MeCN | 40 | >95∶5 |
| 6 | Pd2(dba)3 | dppf | MeCN | 36 | >95∶5 |
| 7 | Pd2(dba)3 | PPh3 | DCE | 95 | >95∶5 |
| 8 | Pd2(dba)3 | PPh3 | EtOAc | 83 | >95∶5 |
| 9 | Pd2(dba)3 | PPh3 | DMF | 62 | >95∶5 |
| 10 | Pd2(dba)3 | PPh3 | Toluene | 82 | >95∶5 |
| 11 | Pd2(dba)3 | PPh3 | THF | 76 | >95∶5 |
| 12b | Pd2(dba)3 | PPh3 | DCE | 86 | >95∶5 |
| 13c | Pd2(dba)3 | PPh3 | DCE | 68 | >95∶5 |

| Entry | Ligand | Solvent | Yield/% | dr | ee/% |
|---|---|---|---|---|---|
| 1 | L1 | MeCN | N.R. | — | — |
| 2 | L2 | MeCN | N.R. | — | — |
| 3 | L3 | MeCN | N.R. | — | — |
| 4 | L4 | MeCN | N.R. | — | — |
| 5 | L5 | MeCN | 80 | >95∶5 | 29 |
| 6 | L6 | MeCN | 23 | >95∶5 | -13 |
| 7 | L7 | MeCN | 67 | >95∶5 | 15 |
| 8 | L8 | MeCN | 88 | >95∶5 | -15 |
| 9 | L9 | MeCN | 62 | >95∶5 | -7 |
| 10 | L10 | MeCN | 72 | >95∶5 | 19 |
| 11 | L11 | MeCN | 41 | >95∶5 | 13 |
| 12 | L12 | MeCN | 25 | >95∶5 | 42 |
| 13 | L12 | DCE | 35 | >95∶5 | 9 |
| 14 | L12 | DMF | 14 | >95∶5 | 12 |
| 15 | L12 | THF | 49 | >95∶5 | 13 |
| 16 | L12 | Toluene | 58 | >95∶5 | 14 |
| Entry | Ligand | Solvent | Yield/% | dr | ee/% |
|---|---|---|---|---|---|
| 1 | L1 | MeCN | N.R. | — | — |
| 2 | L2 | MeCN | N.R. | — | — |
| 3 | L3 | MeCN | N.R. | — | — |
| 4 | L4 | MeCN | N.R. | — | — |
| 5 | L5 | MeCN | 80 | >95∶5 | 29 |
| 6 | L6 | MeCN | 23 | >95∶5 | -13 |
| 7 | L7 | MeCN | 67 | >95∶5 | 15 |
| 8 | L8 | MeCN | 88 | >95∶5 | -15 |
| 9 | L9 | MeCN | 62 | >95∶5 | -7 |
| 10 | L10 | MeCN | 72 | >95∶5 | 19 |
| 11 | L11 | MeCN | 41 | >95∶5 | 13 |
| 12 | L12 | MeCN | 25 | >95∶5 | 42 |
| 13 | L12 | DCE | 35 | >95∶5 | 9 |
| 14 | L12 | DMF | 14 | >95∶5 | 12 |
| 15 | L12 | THF | 49 | >95∶5 | 13 |
| 16 | L12 | Toluene | 58 | >95∶5 | 14 |
| [1] |
For reviews: (a)
doi: 10.1039/C1CS15156H pmid: 29560980 |
|
(b)
doi: 10.1002/adsc.v355.6 pmid: 29560980 |
|
|
(c)
doi: 10.1021/cs401172r pmid: 29560980 |
|
|
(d)
doi: 10.1002/adsc.v360.2 pmid: 29560980 |
|
|
(e)
doi: 10.1039/C6CS00825A pmid: 29560980 |
|
|
(f)
doi: 10.1039/C7OB02686B pmid: 29560980 |
|
|
(g)
doi: 10.1039/C8CC02364F pmid: 29560980 |
|
|
(h)
doi: 10.1021/acs.accounts.0c00697 pmid: 29560980 |
|
|
(i)
doi: 10.1021/acs.accounts.2c00764 pmid: 29560980 |
|
|
(j)
doi: 10.1021/acs.accounts.7b00377 pmid: 29560980 |
|
| [2] |
For recent examples: (a)
doi: 10.1016/j.chempr.2022.06.014 |
|
(b)
|
|
|
(c)
doi: 10.1039/D4QO01424C |
|
|
(d)
doi: 10.1021/acscatal.4c05266 |
|
|
(e)
|
|
|
(f)
|
|
|
(g)
doi: 10.6023/cjoc202501015 |
|
|
(陈刚, 陈东, 聂广杰, 李林轩, 姚辉, 王能中, 黄年玉, 有机化学, 2025, 45, 2139.)
doi: 10.6023/cjoc202501015 |
|
|
(h)
doi: 10.6023/cjoc202500004 |
|
|
(戈丁浩, 石枫, 有机化学, 2025, 45, 717.)
doi: 10.6023/cjoc202500004 |
|
|
(i)
doi: 10.6023/cjoc202406001 |
|
|
(j)
doi: 10.6023/A25050172 |
|
|
(张杰豪, 徐明金, 古满珍, 马浩文, 蔡倩, 化学学报, 2025, 83, 667.)
|
|
|
(k)
doi: 10.6023/A24060191 |
|
|
(王玉, 于聪, 夏艳芳, 林蓝, 陈强, 王新波, 化学学报 2024, 82, 914.)
doi: 10.6023/A24060191 |
|
| [3] |
(a)
pmid: 26163220 |
|
(b)
doi: 10.1002/hlca.v87:6 pmid: 26163220 |
|
|
(c)
doi: 10.1039/C4OB02459A pmid: 26163220 |
|
|
(d)
doi: 10.1016/j.ejmech.2015.06.050 pmid: 26163220 |
|
| [4] |
(a)
doi: 10.1021/ol034646i |
|
(b)
doi: 10.1021/jo035058l |
|
| [5] |
(a)
doi: 10.1002/chem.v24.15 |
|
(b)
doi: 10.1021/acs.joc.3c01315 |
|
| [6] |
For reviews: (a)
doi: 10.1021/ar010105m pmid: 21999240 |
|
(b)
doi: 10.1021/jo201789k pmid: 21999240 |
|
|
(c)
doi: 10.1002/chem.v18.30 pmid: 21999240 |
|
|
(d)
doi: 10.1039/C8CS00274F pmid: 21999240 |
|
|
(e)
doi: 10.1021/acs.accounts.2c00486 pmid: 21999240 |
|
| [7] |
For reviews: (a)
doi: 10.1021/ar500330x |
|
(b)
doi: 10.3390/molecules200711733 |
|
|
(c)
doi: 10.1038/s44160-022-00072-x |
|
|
(d)
|
|
|
(王海清, 杨爽, 张宇辰, 石枫, 有机化学, 2023, 43, 974.)
doi: 10.6023/cjoc202211022 |
|
|
(e)
|
|
|
(杨爽, 王宁宜, 杭青青, 张宇辰, 石枫, 化学学报, 2023, 81, 793.)
doi: 10.6023/A23040192 |
|
| [8] |
For early examples: (a)
doi: 10.1021/ol802029e pmid: 18850717 |
|
(b)
doi: 10.1002/adsc.v351:17 pmid: 18850717 |
|
| [9] |
For recent examples: (a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
doi: 10.1021/acscatal.3c02851 |
|
|
(e)
doi: 10.1039/D4QO00141A |
|
|
(f)
doi: 10.1039/D4QO00148F |
|
|
(g)
doi: 10.1021/acs.joc.4c01080 |
|
|
(h)
doi: 10.1021/acs.orglett.5c02145 |
|
| [10] |
(a)
doi: 10.1021/acs.orglett.2c01137 |
|
(b)
|
|
| [11] |
(a)
doi: 10.1021/acs.orglett.3c02242 |
|
(b)
doi: 10.1021/acs.orglett.3c01883 |
|
|
(c)
doi: 10.1021/acs.joc.3c00707 |
|
|
(d)
doi: 10.1016/j.cclet.2024.109688 |
|
|
(e)
doi: 10.1021/acs.orglett.4c01003 |
|
| [12] |
doi: 10.1039/D3SC00112A |
| [13] |
For reviews: (a)
doi: 10.1021/cr050011g |
|
(b)
doi: 10.1021/acs.accounts.0c00113 |
|
|
(c)
doi: 10.1002/cjoc.v38.12 |
|
|
(d)
doi: 10.1021/acscatal.1c00081 |
|
|
(e)
doi: 10.1002/cjoc.v39.12 |
|
|
(f)
|
|
|
(g)
doi: 10.1007/s11426-023-1683-9 |
|
|
(h)
doi: 10.1021/jacs.2c12995 |
|
|
(i)
doi: 10.1016/j.fmre.2022.07.008 |
|
|
(j)
doi: 10.1021/acs.joc.5c00104 |
|
| [14] |
For reviews: (a)
|
|
(b)
doi: 10.1039/D4QO00515E |
|
| [15] |
For recent examples: (a)
doi: 10.1016/j.fmre.2022.01.002 |
|
(b)
doi: 10.1002/cjoc.v41.1 |
|
|
(c)
doi: 10.1007/s11426-023-1927-3 |
|
|
(d)
doi: 10.6023/cjoc202405040 |
|
|
(e)
doi: 10.1007/s11426-024-2472-2 |
|
| [16] |
(a)
|
|
(游书力, 朱霞珍, 候雪龙, 戴立信, 化学学报, 2001, 59, 1667.)
|
|
|
(b)
doi: 10.1021/cr020027w |
|
|
(c)
doi: 10.1039/C5CS00144G |
|
|
(d)
doi: 10.6023/cjoc201704034 |
|
|
(邓颖颍, 杨文, 杨新, 杨定乔, 有机化学 2017, 37, 3039.)
doi: 10.6023/cjoc201704034 |
|
|
(e)
doi: 10.6023/A18060237 |
|
|
(张毛毛, 骆元元, 陆良秋, 肖文精, 化学学报 2018, 76, 838.)
doi: 10.6023/A18060237 |
|
| [17] |
|
| [18] |
(a)
doi: 10.1021/ja409669r |
|
(b)
doi: 10.1021/acs.accounts.9b00029 |
| [1] | 赵雅宸, 俞飚. Michael/aldol串联环化反应在天然产物合成中的应用[J]. 化学学报, 2025, 83(11): 1397-1413. |
| [2] | 黄涎廷, 韩洪亮, 肖婧, 王帆, 柳忠全. I2O5/KSCN介导的炔烃碘硫氰化反应[J]. 化学学报, 2024, 82(1): 5-8. |
| [3] | 杨爽, 王宁宜, 杭青青, 张宇辰, 石枫. 邻羟基苯基取代的对亚甲基苯醌参与的催化不对称反应研究进展★[J]. 化学学报, 2023, 81(7): 793-808. |
| [4] | 黄家翩, 刘飞, 吴劼. 二氟环丙烯参与的有机反应研究进展★[J]. 化学学报, 2023, 81(5): 520-532. |
| [5] | 许健, 张世樊, 罗莹, 张荔, 张帆, 黄挺菁, 宋秋玲. 自由基促进硫甲基取代的炔酮的环化反应[J]. 化学学报, 2019, 77(9): 932-938. |
| [6] | 田亚伟, 周刚, 赵晓明, 淡文彦. 2-氨基吡嗪衍生物在水相中的选择性氟化反应研究[J]. 化学学报, 2018, 76(12): 962-966. |
| [7] | 蔡忠建, 陆新谋, 汪顺义, 纪顺俊. 铜(I)催化条件下酮肟衍生物与苄烯丙二腈的环化反应构建2-氨基吡啶[J]. 化学学报, 2014, 72(8): 914-919. |
| [8] | 周容, 肖微, 尹祥, 詹固, 陈应春. 环状烯酮与环状1-氮杂二烯的非对映及对映选择性[4+2]环加成反应[J]. 化学学报, 2014, 72(7): 862-866. |
| [9] | 孙利辉, 梁志钦, 叶松. 氮杂环卡宾催化烯酮与氰基查尔酮的[4+2]不对称环加成反应[J]. 化学学报, 2014, 72(7): 841-844. |
| [10] | 张琦, 刘鹤华, 伍贻康. 两个单环有机过氧化物的合成[J]. 化学学报, 2007, 65(17): 1869-1874. |
| [11] | 武芳卉,周峥嵘,王全瑞,丁宗彪,陶凤岗,夏燚,徐伟,华中一. 新型有机电双稳态材料的设计和性质[J]. 化学学报, 2002, 60(1): 4-6. |
| [12] | 黄敏,张熊禄,黄海洪,陈庆华. 手性螺-环丙烷化合物中手性辅基的区域性转换反应的研究[J]. 化学学报, 2001, 59(11): 2000-2006. |
| [13] | 范俊发,尹标林,张瑜峰,吴毓林,伍贻康. 茼蒿素类似物的分子多样性2-(Z)-亚苄基-1,6,9-三氧杂螺环 [4,5-癸-3-烯类化 合物的合成[J]. 化学学报, 2001, 59(10): 1756-1762. |
| [14] | 王平珍,涂永强,李心. 具有抗HIV活性的螺环缩酮类化合物合成研究: Didemnaketals C(1)~C(8)片段 的合成[J]. 化学学报, 2000, 58(4): 458-463. |
| [15] | 黄慧,陈庆华. 光学活性螺-环丙烷双内酯化合物的合成[J]. 化学学报, 2000, 58(2): 248-252. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||