化学学报 ›› 2011, Vol. 69 ›› Issue (15): 1789-1794. 上一篇 下一篇
研究论文
段文胜, 刘斌, 孙占国, 杨斌盛*
DUAN Wen-Sheng, LIU Bin, SUN Zhan-Guo, YANG Bin-Sheng
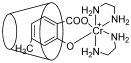
合成了一种新型的4-甲基-水杨酸铬(III)配合物[Cr(III)(4-Me-SA)(en)2]Cl•0.5CH3OH (4-Me-SA=4-甲基-水杨酸, en=乙二胺), 利用X射线晶体衍射和元素分析对其进行了结构表征. 通过紫外-可见吸收光谱、荧光光谱、电导率等方法, 研究了β-环糊精对两种铬配合物[Cr(III)(4-Me-SA)(en)2]Cl•0.5CH3OH (I)和[Cr(III)(SA)(en)2]Cl (II) (SA=水杨酸)的包合行为, 求取了不同温度下的包合常数, 推断了包合反应的主要驱动力等. 结果表明: 298 K时β-环糊精与两种铬配合物均发生了自发包合作用, 形成1∶1型超分子包合物, 疏水性基团甲基的存在有利于包合反应的进行. 与EDTA的竞争反应表明包合物的形成使铬配合物的稳定性增加.