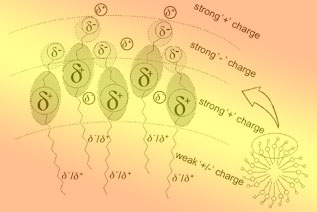
| 1 Fang, Y. Zwitterionic Surfactant, China Light Industry Press, Beijing, 2001 (in Chinese).(方云, 两性表面活性剂, 中国轻工业出版社, 北京,2001.)2 Hou, J. R.; Liu, Z. C.; Zhang, S. F.; Yue, X. A.; Yang, J. Z.J. Petrol. Sci. Eng. 2005, 47, 219.3 Wang, G.; Wang, D. M.; Xia, H. F.; Ju, Y.; Liu, C. D. Acta Petrol. Sinica 2007, 28, 86 (in Chinese).(王刚, 王德民, 夏惠芬, 鞠野, 刘春德, 石油学报, 2007,28, 86.)4 Xia, H. F.; Wang, H. F.; Wang, G.; Hu, J. Q.; Liu, C. D. J China Univ. Petrol. 2007, 31, 74 (in Chinese).(夏惠芬, 王海峰, 王刚, 胡锦强, 刘春德, 中国石油大学学报, 2007, 31, 74.)5 Xia, H. F.; Liu, R. Q.; Ju, Y.; Liu, C. D. J. Daqing Petroleum Institute 2006, 30, 24 (in Chinese).(夏惠芬, 刘仁强, 鞠野, 刘春德, 王亚婷, 大庆石油学院学报, 2006, 30, 24.)6 Schönherr, H.; Rozkiewica, D. I.; Vancso, G. J. Langmuir 2004, 20, 7308.7 Kepczyński, M.; Lewandowska, J.; Romek, M.; Zapotoczny,S.; Ganachaud, F.; Nowakowska, M. Langmuir 2007,23, 7314.8 Jorge, M. Langmuir 2008, 24, 5714.9 Miller, C. A.; Abbott, N. L.; de Pablo, J. J. Langmuir 2009,25, 2811.10 Chen, M. L.; Wang, Z. W.; Wang, H. J.; Zhang, G. X.; Tao,F. M. Chin. Sci. Bull. 2007, 52, 521 (in Chinese).(陈美玲, 王正武, 王海军, 张革新, 陶福明, 科学通报,2007, 52, 521.)11 Gadre, S. R.; Pingale, S. S. Chem. Commun. 1996, (5), 595.12 Yan, P.; Xiao, J. X. Colloids Surf. A 2004, 244, 39.13 Hu, X.; Li, Y.; Sun, H.; Song, X.; Li, Q.; Cao, X.; Li, Z. J.Phys. Chem. B 2010, 114, 8910.14 Huibers, P. D. T. Langmuir 1999, 15, 7546.15 Milani, A.; Castiglioni, C. J. Phys. Chem. A 2010, 114, 624.16 Shishkin, M.; Ziegler, T. J. Phys. Chem. C 2009, 113,21667.17 Karwowski, B. T. J. Mol. Struct.-Theochem. 2009, 915, 73.18 Valiron, P.; Mayer, I. Chem. Phys. Lett. 1997, 275, 46.19 Wallrapp, F.; Voityuk, A.; Guallar, V. J. Chem. Theory Comput. 2009, 5, 3312.20 Niehaus, T. A. J. Mol. Struct.-Theochem 2009, 914, 38.21 Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; et al.Gaussian 03, Revision B.05, Pittsburgh, PA, Gaussian Inc.,2003.22 Connolly, M. L. Science 1983, 221, 709.23 Connolly, M. L. J. Appl. Crystallogr. 1983, 16, 548.24 Florenzano, F. H.; Dias, L. G. Langmuir 1997, 13, 5756.25 Rosen, M. J. Surfactants and Interfacial Phenomena, 3rd Ed., Wiley, New York, 2004.26 Cao, X. L.; Lv, K.; Cui, X. H.; Shi, J.; Yuan, S. L. Acta Phys. Chim. Sin. 2010, 26, 1959 (in Chinese).(曹绪龙, 吕凯, 崔晓红, 石静, 苑世领, 物理化学学报,2010, 26, 1959.)27 Kalyanasundaram, K. Photochemistry in Microheterogeneous Systems, Academic Press, New York, 1987. |