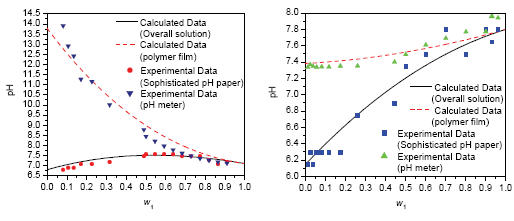
| [1] Gao, X.; Fan, J.; Wang, X. L.; Zhang, Y. S. Acta Chim. Sinica 2013, 71(10), 1411. (高霞, 樊静, 王小龙, 张艳树, 化学学报, 2013, 71(10), 1411.)[2] Huang, B.; Li, Z.; Wang, Y.; Zhang, Y.; Fang, Y. Acta Chim. Sinica 2008, 66(15), 1837. (黄宝华, 黎子进, 汪艳飞, 张煜, 方岩雄, 化学学报, 2008, 66(15), 1837.)[3] Huo, C. D.; Wang, C. Chin. J. Org. Chem. 2013, 33, 2108. (霍聪德, 王程, 有机化学, 2013, 33, 2108.)[4] Zhang, P.; Du, J.; Yang, F.; Zou, G.; Tang, J. Chin. J. Chem. 2005, 23(5), 581.[5] Yokozeki, A.; Shiflett, M. B. Appl. Energy 2007, 84(12), 1258.[6] Shiflett, M. B.; Yokozeki, A. US 20060197053, A1 2006 [Chem. Abstr. 2006, 2597199 A1].[7] Wei, Z.; Wu, X. H.; Zheng, D. X.; Wang, J. Z.; Dong, L. J. Beijing Univ. Chem. Technol. (Nat. Sci. Ed.) 2010, 37(01), 9. (魏治, 武向红, 郑丹星, 王建召, 董丽, 北京化工大学学报(自然科学版), 2010, 37(01), 9.)[8] Tian, T.; Zheng, D. X.; Wu, X. H.; Jiang, Y. R. J. Beijing Univ. Chem. Technol. (Nat. Sci. Ed.) 2008, 35(03), 27. (田涛, 郑丹星, 武向红, 蒋翼然, 北京化工大学学报(自然科学版), 2008, 35(03), 27.)[9] Dong, L.; Zheng, D. X.; Wu, X. H.; Li, F.; Wei, Z. J. Eng. Thermophys. 2009, 30(08), 1271. (董丽, 郑丹星, 武向红, 李枫, 魏治, 工程热物理学报, 2009, 30(08), 1271.)[10] Guan, T. T.; Sun, L.; Huangfu, L. X.; Guo, K. H. Chinese J. Low Temp. Phys. 2011, 33(03), 194. (关婷婷, 孙立, 皇甫立霞, 郭开华, 低温物理学报, 2011, 33(03), 194.)[11] Sun, L.; Guo, K. H.; Huangfu, L. X. Chinese J. Low Temp. Phys. 2011, 33(05), 381. (孙立, 郭开华, 皇甫立霞, 低温物理学报, 2011, 33(05), 381.)[12] Yang, T.; Bi, Y.; Guo, K. H. CIESC J. 2012, 63(10), 3152. (阳涛, 毕崟, 郭开华, 化工学报, 2012, 63(10), 3152.)[13] Yuan, X.; Zhang, S.; Liu, J.; Lu, X. Fluid Phase Equilib. 2007, 257(2), 195.[14] Pan, S. F.; Hu, G. X.; Lv, Y.; Zou, J. W.; Yu, Q. S. Acta Phys.-Chim. Sin. 2010, 26(9), 2494. (潘善飞, 胡桂香, 吕杨, 邹建卫, 俞庆森, 物理化学学报, 2010, 26(9), 2494.)[15] Liang, R.; Yang, M. R.; Zhou, Q. X. Acta Phys.-Chim. Sin. 2010, 26(6), 1468. (梁蕊, 杨美荣, 周庆祥, 物理化学学报, 2010, 26(6), 1468.)[16] Aggarwal, V. K.; Emme, I.; Mereu, A. Chem. Commun. 2002, 1612.[17] Seddon, K. R. J. Chem. Technol. Biot. 1997, 68(4), 351.[18] Fei, Z.; Zhao, D.; Geldbach, T. J.; Scopelliti, R.; Dyson, P. J. Chem. -Eur. J. 2004, 10(19), 4886.[19] Thomazeau, C.; Olivier-Bourbigou, H.; Magna, L.; Luts, S.; Gilbert, B. J. Am. Chem. Soc. 2003, 125(18), 5264.[20] Yang, Y.; Kou, Y. Chem. Commun. 2004, 226.[21] Chen, X. W.; Song, H. B.; Chen, P.; Wang, F. R.; Qian, Y.; Li, X. H. Acta Chim. Sinica. 2012, 70, 770. (陈学伟, 宋红兵, 陈鹏, 王芙蓉, 钱宇, 李雪辉, 化学学报, 2012, 70, 770.)[22] Ober, C. A.; Gupta, R. B. Ind. Eng. Chem. Res. 2012, 51(6), 2524.[23] Guo, K.; Bi, Y.; Sun, L.; Su, H.; Hungpu, L. J. Chem. Eng. Data 2012, 57(8), 2243.[24] Su, H.; Guo, K. H.; Huangfu, L. X.; Sun, L. J. Refrig. 2013, 34(3), 24. (粟航, 郭开华, 皇甫立霞, 孙立, 制冷学报, 2013, 34(3), 24.)[25] Guo, K.; Bi, Y.; Sun, L.; Su, H.; Hungpu, L. J. Chem. Eng. Data 2012, 57(8), 2243.[26] Singh, T.; Kumar, A. Colloids Surf. A 2008, 318(1), 263[27] Fu, S. Z.; Chen, Q. D.; Shen, X. H. Acta Phys.-Chim. Sin. 2011, 27(8), 1913. (付素珍, 陈庆德, 沈兴海, 物理化学学报, 2011, 27(8), 1913.)[28] Sun, L.; Guo, K. H.; Huangfu, L. X. Chin. J. Low Temp. Phys. 2011, 33(6), 467. (孙立, 郭开华, 皇甫立霞, 低温物理学报, 2011, 33(6), 467.)[29] Long, Y. H.; Xiang, M. L.; Gao, Y. H. J. Chongqing Institute Technol. 2001, 15(05), 98. (龙彦辉, 向明礼, 高彦荷, 重庆工学院学报, 2001, 15(05), 98.)[30] http://www.chem.wisc.edu/areas/reich/pkatable/index.htm, 2013.7.22[Z].[31] Hou, H.; Huang, Y. R.; Wang, S. Z.; Bai, B. F. Acta Phys.-Chim. Sin. 2011, 27(11), 2512. (侯海云, 黄银蓉, 王升泽, 白博峰, 物理化学学报, 2011, 27(11), 2512.)[32] Ning, H.; Hou, M.; Mei, Q.; Liu, Y.; Yang, D.; Han, B. Sci. China Chem. 2012, 55(08), 1509. |