化学学报 ›› 2019, Vol. 77 ›› Issue (9): 866-873.DOI: 10.6023/A19040135 上一篇 下一篇
所属专题: 有机自由基化学
研究通讯
杨启亮ab, 王向阳c, 翁信军b, 杨祥b, 徐学涛c, 童晓峰a, 方萍b, 伍新燕a*( ), 梅天胜b*(
), 梅天胜b*( )
)
投稿日期:2019-04-19
发布日期:2019-05-08
通讯作者:
伍新燕,梅天胜
E-mail:xinyanwu@ecust.edu.cn;mei7900@sioc.ac.cn
基金资助:
Yang, Qi-Liangab, Wang, Xiang-Yangc, Weng, Xin-Junb, Yang, Xiangb, Xu, Xue-Taoc, Tong, Xiaofenga, Fang, Pingb, Wu, Xin-Yana*( ), Mei, Tian-Shengb*(
), Mei, Tian-Shengb*( )
)
Received:2019-04-19
Published:2019-05-08
Contact:
Wu, Xin-Yan,Mei, Tian-Sheng
E-mail:xinyanwu@ecust.edu.cn;mei7900@sioc.ac.cn
Supported by:文章分享

芳香族卤代物是非常重要的合成砌块, 卤化反应是有机合成中最基本也是最重要的反应之一. 本工作利用2-(吡啶基)异丙基胺(PIP胺)作为双齿导向基团, 以LiCl作为卤素来源, 通过电化学阳极氧化的策略成功实现了钯催化的芳烃邻位C(sp 2)—H键的氯代反应. 此反应条件官能团耐受性强, 底物适用范围广, 同时能兼容噻吩等杂芳环类底物, 为合成(杂)芳基氯代物提供了一种简洁高效的方法. 该反应可以安全的放大到克级制备, 显示了潜在的工业应用前景. 通过连续的邻位碳氢键溴代和氯代反应还能得到高度复杂的2,5,6-三取代的苯甲酰胺类化合物.
杨启亮, 王向阳, 翁信军, 杨祥, 徐学涛, 童晓峰, 方萍, 伍新燕, 梅天胜. 电氧化促进的钯催化的芳烃C(sp 2)—H键氯代反应[J]. 化学学报, 2019, 77(9): 866-873.
Yang, Qi-Liang, Wang, Xiang-Yang, Weng, Xin-Jun, Yang, Xiang, Xu, Xue-Tao, Tong, Xiaofeng, Fang, Ping, Wu, Xin-Yan, Mei, Tian-Sheng. Palladium-Catalyzed ortho-Selective C—H Chlorination of Arenes Using Anodic Oxidation[J]. Acta Chimica Sinica, 2019, 77(9): 866-873.
| Entry | Variation from standard conditions above | Yieldb/% |
|---|---|---|
| 1 | none | 92 (85)c |
| 2 | PdCl2 instead of Pd(OAc)2 | 89 |
| 3 | Pd(OCOCF3)2 instead of Pd(OAc)2 | 91 |
| 4 | DMA instead of DMF (anode) | 80 |
| 5 | HMPA instead of DMF (anode) | 63 |
| 6 | DMSO instead of DMF (anode) | 12 |
| 7 | H2O instead of DMF (anode) | 34 |
| 8 | HCl instead of LiCl | 72 |
| 9 | NaCl instead of LiCl | 71 |
| 10 | NH4Cl instead of LiCl | 65 |
| 11 | 80 ℃ instead of 90 ℃ | 90 |
| 12 | 100 ℃ instead of 90 ℃ | 92 |
| 13 | 2.5 mA instead of 5 mA (24 h) | 88 |
| 14 | 10 mA instead of 5 mA (6 h) | 67 |
| 15 | no Pd(OAc)2 | nr |
| 16 | no electric current | nr |
| Entry | Variation from standard conditions above | Yieldb/% |
|---|---|---|
| 1 | none | 92 (85)c |
| 2 | PdCl2 instead of Pd(OAc)2 | 89 |
| 3 | Pd(OCOCF3)2 instead of Pd(OAc)2 | 91 |
| 4 | DMA instead of DMF (anode) | 80 |
| 5 | HMPA instead of DMF (anode) | 63 |
| 6 | DMSO instead of DMF (anode) | 12 |
| 7 | H2O instead of DMF (anode) | 34 |
| 8 | HCl instead of LiCl | 72 |
| 9 | NaCl instead of LiCl | 71 |
| 10 | NH4Cl instead of LiCl | 65 |
| 11 | 80 ℃ instead of 90 ℃ | 90 |
| 12 | 100 ℃ instead of 90 ℃ | 92 |
| 13 | 2.5 mA instead of 5 mA (24 h) | 88 |
| 14 | 10 mA instead of 5 mA (6 h) | 67 |
| 15 | no Pd(OAc)2 | nr |
| 16 | no electric current | nr |
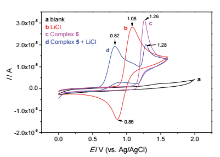
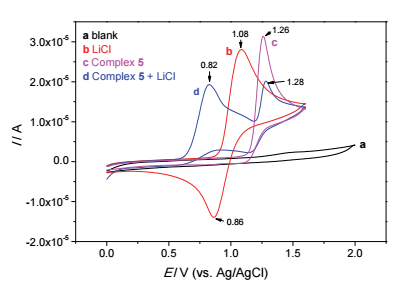
| [1] |
(a) Butler, A.; Walker, J. V. Chem. Rev. 1993, 93, 1937
doi: 10.1002/(ISSN)1521-3773 |
|
(b) Nicolaou, K. C.; Bulger, P. G.; Sarlah, D. Angew Chem., Int. Ed. 2005, 44, 4442.
doi: 10.1002/(ISSN)1521-3773 |
|
| [2] |
For selected reviews see: (a) Hassan, J.; Se'vignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M . Chem. Rev. 2002, 102, 1359
doi: 10.1021/cr000664r |
|
(b) Littke, A. F.; Fu, G. C. Angew Chem., Int. Ed. 2002, 41, 4176;
doi: 10.1021/cr000664r |
|
|
(c) Corbet, J. P.; Mignani, G . Chem. Rev. 2006, 106, 2651;
doi: 10.1021/cr000664r |
|
|
(d) Yin, L.-X.; Liebscher, J . Chem. Rev. 2007, 107, 133.
doi: 10.1021/cr000664r |
|
| [3] |
For a review on an ortho-lithiation approach, see: Snieckus, V. Chem. Rev . 1990, 90, 879.
doi: 10.1021/cr00104a001 |
| [4] |
Hodgson, H. H. Chem. Rev. 1947, 40, 251.
doi: 10.1021/cr60126a003 |
| [5] | De La Mare, P. B. D . Electrophilic Halogenation, Cambridge University Press, New York, 1976. |
| [6] |
For selected reviews on transition-metal-catalyzed C—H functionalization, see: (a) Daugulis, O.; Do, H.-Q.; Shabashov, D. Acc. Chem. Res. 2009, 42, 1074
doi: 10.1021/ar9000058 |
|
(b) Chen, X.; Engle, K. M.; Wang, D.-H.; Yu, J.-Q. Angew Chem., Int. Ed. 2009, 48, 5094;
doi: 10.1021/ar9000058 |
|
|
(c) Giri, R.; Shi, B.-F.; Engle, K. M.; Maugel, N.; Yu, J.-Q . Chem. Soc. Rev. 2009, 38, 3242;
doi: 10.1021/ar9000058 |
|
|
(d) Lyons, T. W.; Sanford, M. S . Chem. Rev. 2010, 110, 1147;
doi: 10.1021/ar9000058 |
|
|
(e) Arockiam, P. B.; Bruneau, C.; Dixneuf, P. H . Chem. Rev. 2012, 112, 5879;
doi: 10.1021/ar9000058 |
|
|
(f) Ackermann, L. C . Acc. Chem. Res. 2014, 47, 281;
doi: 10.1021/ar9000058 |
|
|
(g) Pei, P.; Zhang, F.; Yi, H.; Lei, A . Acta Chim. Sinica 2017, 75, 15 (in Chinese).
doi: 10.1021/ar9000058 |
|
|
( 裴朋昆, 张凡, 易红, 雷爱文, 化学学报, 2017, 75, 15);
doi: 10.1021/ar9000058 |
|
|
(h) Du, J.-Y.; Xia, C.-G.; Sun, W . Acta Chim. Sinica 2018, 76, 329 (in Chinese).
doi: 10.1021/ar9000058 |
|
|
( 杜俊毅, 夏春谷, 孙伟, 化学学报, 2018, 76, 329).
doi: 10.1021/ar9000058 |
|
| [7] |
For selected examples of palladium-catalyzed direct halogenation of C—H bonds, see: (a) Dick, A. R.; Hull, K. L.; Sanford, M. S. J. Am. Chem. Soc. 2004, 126, 2300
doi: 10.1021/ja031543m |
|
(b) Wan, X. B.; Ma, Z. X.; Li, B. J.; Zhang, K. Y.; Cao, S. K.; Zhang, S. W.; Shi, Z. J. J. Am. Chem. Soc. 2006, 128, 7416;
doi: 10.1021/ja031543m |
|
|
(c) Zhao, X.; Dimitrijevic, E.; Dong, V. M . J. Am. Chem. Soc. 2009, 131, 3466;
doi: 10.1021/ja031543m |
|
|
(d) Wang, X.-C.; Hu, Y.; Bonacorsi, S.; Hong, Y.; Burrell, R.; Yu, J.-Q . J. Am. Chem. Soc. 2013, 135, 10326;
doi: 10.1021/ja031543m |
|
|
(e) Gao, D.; Gu, Q.; You, S.-L . ACS Catal. 2014, 4, 2741;
doi: 10.1021/ja031543m |
|
|
(f) Chu, L.; Xiao, K.-J.; Yu, J.-Q . Science 2014, 346, 451;
doi: 10.1021/ja031543m |
|
|
(g) Zhao, K.; Yang, L.; Liu, J.-H.; Xia, C.-G . Chin. J. Org. Chem. 2018, 38, 2833 (in Chinese).
doi: 10.1021/ja031543m |
|
|
( 赵康, 杨磊, 刘建华, 夏春谷, 有机化学, 2018, 38, 2833).
doi: 10.1021/ja031543m |
|
| [8] |
(a) Chen, X.; Hao, X.-S.; Goodhue, C. E.; Yu, J.-Q. J. Am. Chem. Soc. 2006, 128, 6790
doi: 10.1021/ja061715q |
|
(b) Wang, W.; Pan, C.; Chen, F.; Cheng, J . Chem. Commun. 2011, 47, 3978;
doi: 10.1021/ja061715q |
|
|
(c) Mo, S.; Zhu, Y.; Shen, Z . Org. Biomol. Chem. 2013, 11, 2756;
doi: 10.1021/ja061715q |
|
|
(d) Du, Z.-J.; Gao, L.-X.; Lin, Y.-J.; Han, F.-S . ChemCatChem 2014, 6, 123;
doi: 10.1021/ja061715q |
|
|
(e) Hufman, L. M.; Stahl, S. S . J. Am. Chem. Soc. 2008, 130, 9196;
doi: 10.1021/ja061715q |
|
|
(f) King, A. E.; Huffman, L. M.; Casitas, A.; Costas, M.; Ribas, X.; Stahl, S. S . J. Am. Chem. Soc. 2010, 132, 12068;
doi: 10.1021/ja061715q |
|
|
(g) Wang, Z.-L.; Zhao, L.; Wang, M.-X . Org. Lett. 2011, 13, 6560;
doi: 10.1021/ja061715q |
|
|
(h) Wang, Z.-L.; Zhao, L.; Wang, M.-X . Org. Lett. 2012, 14, 1472;
doi: 10.1021/ja061715q |
|
|
(i) Casitas, A.; Ribas, X . Chem. Sci. 2013, 4, 2301;
doi: 10.1021/ja061715q |
|
|
(j) Zhang, H.; Yao, B.; Zhao, L.; Wang, D.-X.; Xu, B.-Q.; Wang, M.-X . J. Am. Chem. Soc. 2014, 136, 6326;
doi: 10.1021/ja061715q |
|
|
(k) Truong, T.; Klimovica, K.; Daugulis, O . J. Am. Chem. Soc. 2013, 135, 9342;
doi: 10.1021/ja061715q |
|
|
(l) Suess, A. M.; Ertem, M. Z. C.; Cramer, J.; Stahl, S. S . J. Am. Chem. Soc. 2013, 135, 9797;
doi: 10.1021/ja061715q |
|
|
(m) Zhang, Q.; Yin, X.-S.; Zhao, S.; Fang, S.-L.; Shi, B.-F . Chem. Commun. 2014, 50, 8353.
doi: 10.1021/ja061715q |
|
| [9] |
For selected examples of rhodium-catalyzed direct halogenation of C—H bonds, see: (a) Schroder, N.; Wencel-Delord, J.; Glorius, F. J. Am. Chem. Soc. 2012, 134, 8298
doi: 10.1021/ja302631j |
|
(b) Hwang, H.; Kim, J.; Jeong, J.; Chang, S. J. Am. Chem. Soc. 2014, 136, 10770;
doi: 10.1021/ja302631j |
|
|
(c) Qian, G.; Hong, X.; Liu, B.; Mao, H.; Xu, B. Org. Lett. 2014, 16, 5294.
doi: 10.1021/ja302631j |
|
| [10] |
For an example of ruthenilum-catalyzed ortho-halogenation, see: Wang, L.-H.; Ackermann, L . Chem. Commun. 2014, 50, 1083.
doi: 10.1039/C3CC47852A |
| [11] |
For an example of Co-catalyzed ortho-halogenation, see: (a) Yu, D.-G.; Gensch, T.; de Azambuja, F.; Vásquez-Céspedes, S.; Glorius, F. J. Am. Chem. Soc. 2014, 136, 17722
doi: 10.1021/ja511011m |
|
(b) Gu, Z.-Y., Ji, S.-J . Acta Chim. Sinica 2018, 76, 347 (in Chinese).
doi: 10.1021/ja511011m |
|
|
( 顾正洋, 纪顺俊, 化学学报, 2018, 76, 347).
doi: 10.1021/ja511011m |
|
| [12] |
For recent reviews on organic electrochemistry, see: (a) Yuan, Y.; Cao, Y.; Qiao, J.; Lin, Y.; Jiang, X.; Weng, Y.; Tang, S.; Lei, A. Chin. J. Chem. 2019, 37, 49
doi: 10.1002/cjoc.v37.1 |
|
(b) Tang, S.; Liu, Y.; Lei, A . Chem 2018, 4, 27;
doi: 10.1002/cjoc.v37.1 |
|
|
(c) Liu, K.; Song, C.; Lei, A . Org. Biomol. Chem. 2018, 16, 2375;
doi: 10.1002/cjoc.v37.1 |
|
|
(d) Sauer, G. S.; Lin, S . ACS Catal. 2018, 8, 5175;
doi: 10.1002/cjoc.v37.1 |
|
|
(e) Parry, J.; Fu, N.; Lin, S . Synlett 2018, 29, 257;
doi: 10.1002/cjoc.v37.1 |
|
|
(f) Nutting, J. E.; Rafiee, M.; Stahl, S. S . Chem. Rev. 2018, 118, 4834;
doi: 10.1002/cjoc.v37.1 |
|
|
(g) Jiang, Y.; Xu, K.; Zeng, C . Chem. Rev. 2018, 118, 4485;
doi: 10.1002/cjoc.v37.1 |
|
|
(h) Waldvogel, S. R.; Lips, S.; Selt, M.; Riehl, B.; Kampf, C . Chem. Rev. 2018, 118, 6706;
doi: 10.1002/cjoc.v37.1 |
|
|
(i) Moeller, K. D . Chem. Rev. 2018, 118, 4817;
doi: 10.1002/cjoc.v37.1 |
|
|
(j) Yang, Q.-L.; Fang, P.; Mei, T.-S . Chin. J. Chem. 2018, 36, 338;
doi: 10.1002/cjoc.v37.1 |
|
|
(k) Yan, M.; Kawamata, Y.; Baran, P. S . Chem. Rev. 2017, 117, 13230;
doi: 10.1002/cjoc.v37.1 |
|
|
(l) Horn, E. J.; Rosen, B. R.; Baran, P. S . ACS Cent. Sci. 2016, 2, 302;
doi: 10.1002/cjoc.v37.1 |
|
|
(m) Hou, Z.-W.; Mao, Z.-Y.; Xu, H.-C . Synlett 2017, 28, 1867;
doi: 10.1002/cjoc.v37.1 |
|
|
(n) Francke, R.; Little, R. D . Chem. Soc. Rev. 2014, 43, 2492.
doi: 10.1002/cjoc.v37.1 |
|
| [13] |
For recent examples on organic electrochemistry, see: (a) Yuan, Y.; Yao, A.; Zheng, Y.; Gao, M.; Zhou, Z.; Qiao, J.; Hu, J.; Ye, B.; Zhao, J.; Wen, H.; Lei, A . iScience 2019, 12, 293
doi: 10.1016/j.isci.2019.01.017 |
|
(b) Wang, P.; Tang, S.; Huang, P. F.; Lei, A. W.; Angew. Chem., Int. Ed. 2017, 56, 3009;
doi: 10.1016/j.isci.2019.01.017 |
|
|
(c) Zhang, Z.; Zhang, L.; Cao, Y.; Li, F.; Bai, G.; Liu, G.; Yang, Y.; Mo, F . Org. Lett. 2019, 21, 762;
doi: 10.1016/j.isci.2019.01.017 |
|
|
(d) Yan, H.; Hou, Z.-W.; Xu, H.-C . Angew. Chem., Int. Ed. 2019, 58, 4592;
doi: 10.1016/j.isci.2019.01.017 |
|
|
(e) Hou, Z.-W.; Mao, Z.-Y.; Zhao, H.-B.; Melcamu, Y. Y.; Lu, X.; Song, J.; Xu, H.-C . Angew. Chem., Int. Ed. 2016, 55, 9168;
doi: 10.1016/j.isci.2019.01.017 |
|
|
(f) Rafiee, M.; Wang, F.; Hruszkewycz, D. P.; Stahl, S. S . J. Am. Chem. Soc. 2018, 140, 22;
doi: 10.1016/j.isci.2019.01.017 |
|
|
(g) Wang, H.; Zhang, J.; Tan, J.; Xin, L.; Li, Y.; Zhang, S.; Xu, K . Org. Lett. 2018, 20, 2505;
doi: 10.1016/j.isci.2019.01.017 |
|
|
(h) . , , Lin, D. Z.; Huang, J. M.; Org. Lett. 2018, 20, 2112;
doi: 10.1016/j.isci.2019.01.017 |
|
|
(i) Ye, Z.; Ding, M.; Wu, Y.; Li, Y.; Hua, W.; Zhang, F . Green Chem. 2018, 20, 1732;
doi: 10.1016/j.isci.2019.01.017 |
|
|
(j) Wang, Q.-Q.; Xu, K.; Jiang, Y.-Y.; Liu, Y.-G.; Sun, B.- G.; Zeng, C.-C . Org. Lett. 2017, 19, 5517;
doi: 10.1016/j.isci.2019.01.017 |
|
|
(k) Wiebe, A.; Lips, S.; Schollmeyer, D.; Franke, R.; Waldvogel, S. R . Angew. Chem., Int. Ed. 2017, 56, 14727;
doi: 10.1016/j.isci.2019.01.017 |
|
|
(l) Kawamata, Y.; Yan, M.; Liu, Z.; Bao, D.-H.; Chen, J.; Starr, J.; Baran, P. S . J. Am. Chem. Soc. 2017, 139, 7448;
doi: 10.1016/j.isci.2019.01.017 |
|
|
(m) Horn, E. J.; Rosen, B. R.; Chen, Y.; Tang, J.; Chen, K.; Eastgate, M. D.; Baran, P. S . Nature 2016, 533, 77.
doi: 10.1016/j.isci.2019.01.017 |
|
| [14] |
For selected reviews on transition-metal-catalyzed electrochemical C—H functionalization, see: (a) Sauermann, N.; Meyer, T. H.; Qiu, Y.; Ackermann, L . ACS Catal. 2018, 8, 7086
doi: 10.1021/acscatal.8b01682 |
|
(b) Sauermann, N.; Meyer, T. H.; Ackermann, L . Chem.-Eur. J. 2018, 24, 16209;
doi: 10.1021/acscatal.8b01682 |
|
|
(c) Ma, C.; Fang, P.; Mei, T.-S . ACS Catal. 2018, 8, 7179;
doi: 10.1021/acscatal.8b01682 |
|
|
(d) Jiao, K.-J.; Zhao, C.-Q.; Fang, P.; Mei, T.-S . Tetrahedron Lett. 2017, 58, 797;
doi: 10.1021/acscatal.8b01682 |
|
|
(e) Wu, Y.-X.; Xi, Y.-C.; Zhao, M.; Wang, S.-Y . Chin. J. Org. Chem. 2018, 38, 2590 (in Chinese).
doi: 10.1021/acscatal.8b01682 |
|
|
( 吴亚星, 席亚超, 赵明, 王思懿, 有机化学, 2018, 38, 2590).
doi: 10.1021/acscatal.8b01682 |
|
| [15] |
For selected examples on transition-metal-catalyzed electrochemical C—H functionalization, see:(a) Qiu, Y.; Stangier, M.; Meyer, T. H.; Oliveira, J. C. A.; Ackermann, L. Angew. Chem. Int. Ed. 2018, 57, 14179
doi: 10.1002/anie.201809611 |
|
(b) Sauermann, N.; Mei, R.; Ackermann, L. Angew. Chem. Int. Ed. 2018, 57, 5090;
doi: 10.1002/anie.201809611 |
|
|
(c) Gao, X.; Wang, P.; Zeng, L.; Tang, S.; Lei, A . J. Am. Chem. Soc. 2018, 140, 4195;
doi: 10.1002/anie.201809611 |
|
|
(d) Tang, S.; Wang, D.; Liu, Y.; Liu, L.; Lei, A . Nature Commun. 2018, 9, 798;
doi: 10.1002/anie.201809611 |
|
|
(e) Xu, F.; Li, Y.-J.; Huang, C.; Xu, H.-C . ACS Catal. 2018, 8, 3820;
doi: 10.1002/anie.201809611 |
|
|
(f) Shrestha, A.; Lee, M.; Dunn, A. L.; Sanford, M. S . Org. Lett. 2018, 20, 204;
doi: 10.1002/anie.201809611 |
|
|
(g) Grayaznova, T. V.; Dudkina, Y. B.; Islamov, D. R.; Kataeva, O. N.; Sinyashin, O. G.; Vicic, D. A.; Budnikova, Y. Н . J. Organomet. Chem. 2015, 785, 68;
doi: 10.1002/anie.201809611 |
|
|
(h) Amatore, C.; Cammoun, C.; Jutand, A . Adv. Synth. Catal. 2007, 349, 292;
doi: 10.1002/anie.201809611 |
|
|
(i) Freund, M. S.; Labinger, J. A.; Lewis, N. S.; Bercaw, J. E . J. Mol. Catal. 1994, 87, L11.
doi: 10.1002/anie.201809611 |
|
| [16] |
Kakiuchi, F.; Kochi, T.; Mutsutani, H.; Kobayashi, N.; Urano, S.; Sato, M.; Nishiyama, S.; Tanabe, T. J. Am. Chem. Soc. 2009, 131, 11310.
doi: 10.1021/ja9049228 |
| [17] |
(a) Yang, Q.-L.; Wang, X.-Y.; Wang, T.-L.; Yang, X.; Liu, D.; Tong, X.; Wu, X.-Y.; Mei, T.-S . Org. Lett. 2019, 21, 2645
doi: 10.1021/acs.orglett.9b00629 |
|
(b) Yang, Q.-L.; Li, C.-Z.; Zhang, L.-W.; Li, Y.-Y.; Tong, X.; Wu, X.-Y.; Mei, T.-S . Organometallics 2019, 38, 1208;
doi: 10.1021/acs.orglett.9b00629 |
|
|
(c) Yang, Q.-L.; Wang, X.-Y.; Lu, J.-Y.; Zhang, L.-P.; Fang, P.; Mei, T.-S . J. Am. Chem. Soc. 2018, 140, 11487;
doi: 10.1021/acs.orglett.9b00629 |
|
|
(d) Li, Y.-Q.; Yang, Q.-L.; Fang, P.; Mei, T.-S.; Zhang, D . Org. Lett. 2017, 19, 2905;
doi: 10.1021/acs.orglett.9b00629 |
|
|
(e) Ma, C.; Zhao, C.-Q.; Li, Y.-Q.; Zhang, L.-P.; Xu, X.; Zhang, K.; Mei, T.-S . Chem. Commun. 2017, 53, 12189;
doi: 10.1021/acs.orglett.9b00629 |
|
|
(f) Yang, Q.-L.; Li, Y.-Q.; Ma, C.; Fang, P.; Zhang, X.-J.; Mei, T.-S . J. Am. Chem. Soc. 2017, 139, 3293.
doi: 10.1021/acs.orglett.9b00629 |
|
| [18] |
During this manuscript preparation, Kakiuchi reported similar work using benzamide derivatives: Konishi, M.; Tsuchida, K.; Sano, K.; Kochi, T.; Kakiuchi, F. J. Org. Chem. 2017, 82, 8716. However, the work was independently carried out. The reaction conditions and directing groups used in these two studies are different.
doi: 10.1021/acs.joc.7b01137 |
| [19] |
(a) Sun, H.; Yu, L.; Jin, X.; Hu, X.; Wang, D.; Chen, G. Z . Electrochem. Commun. 2005, 7, 685
doi: 10.1016/j.elecom.2005.04.020 |
|
(b) Yu, L.; Jin, X.; Chen, G. Z. J. Electroanal. Chem. 2013, 688, 371.
doi: 10.1016/j.elecom.2005.04.020 |
| [1] | 黄家翩, 刘飞, 吴劼. 二氟环丙烯参与的有机反应研究进展★[J]. 化学学报, 2023, 81(5): 520-532. |
| [2] | 韩明亮, 徐丽华. 过渡金属催化的硫酯的交叉偶联反应研究进展[J]. 化学学报, 2023, 81(4): 381-392. |
| [3] | 韩叶强, 史炳锋. 钯(II)催化不对称C(sp3)—H键官能团化研究进展★[J]. 化学学报, 2023, 81(11): 1522-1540. |
| [4] | 刘霞, 匡春香, 苏长会. 过渡金属催化的1,2,3-三氮唑导向的C—H键官能团化反应研究进展[J]. 化学学报, 2022, 80(8): 1135-1151. |
| [5] | 王金格, 周伟, 李佳轶, 丁雅妮, 高继慧. 脉冲电催化的研究进展及性能强化机制[J]. 化学学报, 2022, 80(11): 1555-1568. |
| [6] | 廖港, 吴勇杰, 史炳锋. 非共价作用在过渡金属催化的选择性碳氢键活化中的应用[J]. 化学学报, 2020, 78(4): 289-298. |
| [7] | 张洪浩, 俞寿云. 过渡金属与光氧化还原协同催化的烯丙基取代反应的研究进展[J]. 化学学报, 2019, 77(9): 832-840. |
| [8] | 路福东, 姜烜, 陆良秋, 肖文精. 炔丙基自由基在有机合成化学中的应用[J]. 化学学报, 2019, 77(9): 803-813. |
| [9] | 王强, 顾庆, 游书力. 过渡金属催化不对称C—H键官能团化反应构建轴手性联芳基化合物研究进展[J]. 化学学报, 2019, 77(8): 690-704. |
| [10] | 周锵, 陆平. 手性路易斯碱和过渡金属协同催化反应的进展[J]. 化学学报, 2018, 76(11): 825-830. |
| [11] | 鲁鸿, 刘金宇, 李红玉, 许鹏飞. 氮杂环卡宾与过渡金属共催化反应研究进展[J]. 化学学报, 2018, 76(11): 831-837. |
| [12] | 张毛毛, 骆元元, 陆良秋, 肖文精. 过渡金属与有机小分子协同催化的不对称烯丙基取代反应研究进展[J]. 化学学报, 2018, 76(11): 838-849. |
| [13] | 李娅琼, 黄志真. 过渡金属催化和有机小分子参与的α,β-不饱和酮与烯丙醇的Morita-Baylis-Hillman反应[J]. 化学学报, 2017, 75(3): 280-283. |
| [14] | 廖港, 史炳锋. 过渡金属催化的惰性碳氢键卤化反应研究进展[J]. 化学学报, 2015, 73(12): 1283-1293. |
| [15] | 鲁平, 冯超, 罗德平. Rh/Ag双金属催化的碳氢键氧化Heck反应研究[J]. 化学学报, 2015, 73(12): 1315-1319. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||