化学学报 ›› 2021, Vol. 79 ›› Issue (11): 1360-1371.DOI: 10.6023/A21070340 上一篇 下一篇
综述
投稿日期:2021-07-23
发布日期:2021-08-23
通讯作者:
奚江波, 陈伟
作者简介: |
黄杰, 2019年本科毕业于武汉工程大学, 目前在武汉工程大学化学与环境工程学院柏正武课题组开展研究工作, 研究方向是碳基催化剂在有机反应中的应用. |
 |
奚江波, 博士、副教授、硕士生导师. 主要研究领域涉及高性能贵金属纳米催化剂、单原子催化剂和无金属碳基催化剂的制备及其在有机催化、电催化、生物传感等方面的应用, 以及催化机理的研究. 近年来在Advanced Functional Materials、Applied Catalysis B: Environmental、Journal of Catalysis等国际期刊上发表了学术论文30余篇, 获授权中国发明专利5项. |
 |
陈伟, 博士, 副教授, 硕士生导师. 主要研究领域涉及无机材料的合成、表征与应用, 并从事手性分离材料的研究及色谱分析工作. 主持湖北省教育厅科学技术研究计划重点项目1项, 在国内外学术期刊上发表科研论文20余篇, 其中SCI收录论文7篇, 授权发明专利1项. |
 |
柏正武, 博士、教授、博士生导师. 研究领域涉及有机合成、药物研制及手性功能材料的制备与应用. 主持并完成了4项国家自然科学基金资助的面上项目, 取得了一些有自主知识产权的研究成果, 获得多项发明专利权, 在国际学术期刊上发表研究论文60余篇. |
基金资助:
Jie Huang, Jiangbo Xi( ), Wei Chen(
), Wei Chen( ), Zhengwu Bai
), Zhengwu Bai
Received:2021-07-23
Published:2021-08-23
Contact:
Jiangbo Xi, Wei Chen
Supported by:文章分享
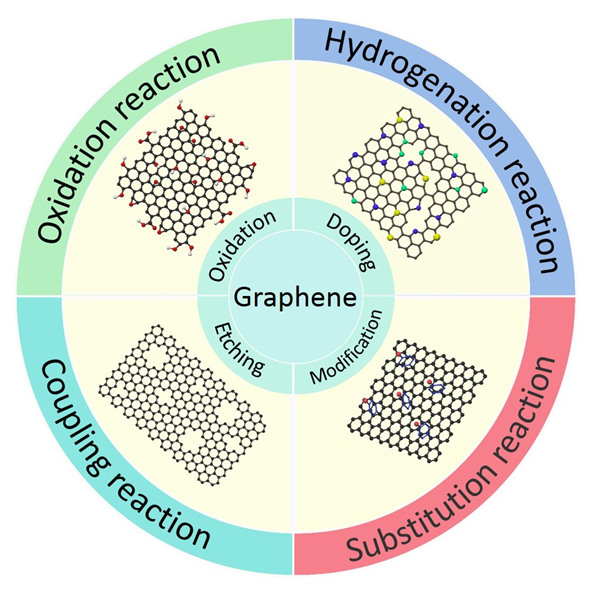
近年来, 随着绿色化学和可持续发展等创新理念的提出, 无金属催化剂逐渐成为催化领域的研究热点和前沿. 石墨烯作为一种新型纳米碳材料, 具有机械强度大、比表面积高、稳定性好、电学性质优异等特点, 经过改性或功能化后的衍生物可以作为无金属碳基催化剂, 在有机反应中展现了良好的应用前景. 本文综述了石墨烯衍生物的结构和性质, 探究了石墨烯基材料的结构与催化活性之间的关系, 总结了此类材料作为无金属催化剂在氧化、还原/氢化、偶联、取代反应以及其他有机反应中的应用和反应机理.
黄杰, 奚江波, 陈伟, 柏正武. 石墨烯衍生物作为无金属碳基催化剂在有机催化中的应用[J]. 化学学报, 2021, 79(11): 1360-1371.
Jie Huang, Jiangbo Xi, Wei Chen, Zhengwu Bai. Graphene-derived Materials for Metal-free Carbocatalysis of Organic Reactions[J]. Acta Chimica Sinica, 2021, 79(11): 1360-1371.




| Catalyst | Preparation method | Functional groups or dopants | Reaction |
|---|---|---|---|
| GO[ | Hummers method | O-containing groups | Oxidation of alcohol |
| abGO[ | GO was treated with sequential base and acid | O-containing groups | |
| NG-T[ | GO annealed under NH3 flow | Doped N atoms | |
| BG[ | GO annealed with boric acid | Doped B atoms (2.9%) | |
| G1000[ | Pyrolysis of the sulfate lignin | Doped S and O atoms | |
| CCG[ | Chemical reduction of GO | π-system of graphene | Oxidation of benzene |
| GO[ | Modified Hummers method | O-containing groups | Oxidation of cyclohexane |
| NGO[ | Modified Hummers method | O-containing groups | Oxidation of alkyl-substituted arenes |
| LC-N[ | CVD method with acetonitrile as N source | Doped N atoms (8.9%) | |
| NG[ | Arc-discharge exfoliation and subsequently annealed with NH3 | Doped N atoms (0.42%) | Oxidation of olefin |
| rGO[ | GO reduction (modified Wallace's method) | The zigzag edges of rGO | Reduction of nitroaromatics |
| NG[ | Hydrothermal treatment of GO-urea mixture and subsequent annealing | Doped N atoms (2.03%) | |
| NHG[ | Hydrothermal treatment of GO-NH3-H2O2 mixture | Doped N atoms(9.77%) | |
| NPG[ | Pyrolysis of a microwave-exfoliated graphite and hexachlorocyclotriphosphazene | Doped N and P atoms | |
| Graphene[ | Pyrolysis of alginate | Lewis acid-base pairs | Selective acetylene hydrogenation |
| GO[ | Modified Hummers method | O-containing groups | Cross-coupling reaction |
| GO[ | — | O-containing groups | |
| Graphite oxide[ | Modified Hummers method | O-containing groups | Oxidative coupling of amines |
| ba-GO[ | GO was treated with base and acid | O-containing groups | |
| PG[ | Annealing of triphenylphosphine | Doped P atoms | |
| BNHG[ | Annealing of of dicyandiamide, glucose and boric acid | Doped B and N atoms | |
| GO[ | Modified Hummers method | O-containing groups | Friedel-Crafts alkylation |
| GO[ | Modified Hummers method | O-containing groups | |
| GO[ | Hummers method | O-containing groups | |
| Graphite[ | — | — | Friedel-Crafts acylation |
| GR-SO3H[ | rGO reacts with sodium nitrite and sulfanilic acid at room temperature | Denzenesulfonic acid groups | Transesterification |
| GO-S[ | Modified Hummers method | Sulfonic acid groups and O-containing groups | Esterification |
| Graphite oxide[ | Hummers method | — | Claisen-Schmidt condensation |
| GO-DETA[ | GO modification with triethylamine | Primary, secondary amino and Oxygen-containing groups | Michael reaction and Knoevenagel reaction |
| GO[ | Modified Hummers method | Oxygen-containing groups | Hydrolysis reaction |
| GO[ | — | O-containing groups | N-formylation reaction |
| GO[ | — | O-containing groups | Dehydrogenation reaction |
| GO[ | — | O-containing groups | Iodization reaction |
| Graphite oxide[ | Hummers method | O-containing groups | Dehydration reaction |
| Graphite[ | — | — |
| Catalyst | Preparation method | Functional groups or dopants | Reaction |
|---|---|---|---|
| GO[ | Hummers method | O-containing groups | Oxidation of alcohol |
| abGO[ | GO was treated with sequential base and acid | O-containing groups | |
| NG-T[ | GO annealed under NH3 flow | Doped N atoms | |
| BG[ | GO annealed with boric acid | Doped B atoms (2.9%) | |
| G1000[ | Pyrolysis of the sulfate lignin | Doped S and O atoms | |
| CCG[ | Chemical reduction of GO | π-system of graphene | Oxidation of benzene |
| GO[ | Modified Hummers method | O-containing groups | Oxidation of cyclohexane |
| NGO[ | Modified Hummers method | O-containing groups | Oxidation of alkyl-substituted arenes |
| LC-N[ | CVD method with acetonitrile as N source | Doped N atoms (8.9%) | |
| NG[ | Arc-discharge exfoliation and subsequently annealed with NH3 | Doped N atoms (0.42%) | Oxidation of olefin |
| rGO[ | GO reduction (modified Wallace's method) | The zigzag edges of rGO | Reduction of nitroaromatics |
| NG[ | Hydrothermal treatment of GO-urea mixture and subsequent annealing | Doped N atoms (2.03%) | |
| NHG[ | Hydrothermal treatment of GO-NH3-H2O2 mixture | Doped N atoms(9.77%) | |
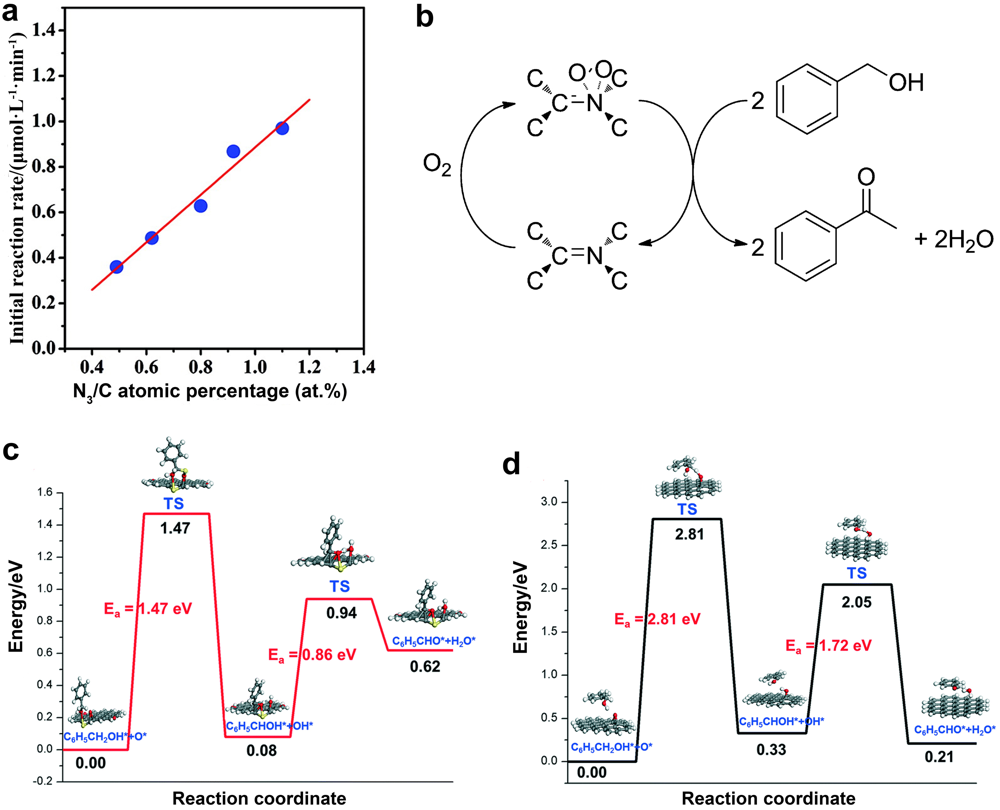
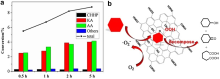
| NPG[ | Pyrolysis of a microwave-exfoliated graphite and hexachlorocyclotriphosphazene | Doped N and P atoms | |
| Graphene[ | Pyrolysis of alginate | Lewis acid-base pairs | Selective acetylene hydrogenation |
| GO[ | Modified Hummers method | O-containing groups | Cross-coupling reaction |
| GO[ | — | O-containing groups | |
| Graphite oxide[ | Modified Hummers method | O-containing groups | Oxidative coupling of amines |
| ba-GO[ | GO was treated with base and acid | O-containing groups | |
| PG[ | Annealing of triphenylphosphine | Doped P atoms | |
| BNHG[ | Annealing of of dicyandiamide, glucose and boric acid | Doped B and N atoms | |
| GO[ | Modified Hummers method | O-containing groups | Friedel-Crafts alkylation |
| GO[ | Modified Hummers method | O-containing groups | |
| GO[ | Hummers method | O-containing groups | |
| Graphite[ | — | — | Friedel-Crafts acylation |
| GR-SO3H[ | rGO reacts with sodium nitrite and sulfanilic acid at room temperature | Denzenesulfonic acid groups | Transesterification |
| GO-S[ | Modified Hummers method | Sulfonic acid groups and O-containing groups | Esterification |
| Graphite oxide[ | Hummers method | — | Claisen-Schmidt condensation |
| GO-DETA[ | GO modification with triethylamine | Primary, secondary amino and Oxygen-containing groups | Michael reaction and Knoevenagel reaction |
| GO[ | Modified Hummers method | Oxygen-containing groups | Hydrolysis reaction |
| GO[ | — | O-containing groups | N-formylation reaction |
| GO[ | — | O-containing groups | Dehydrogenation reaction |
| GO[ | — | O-containing groups | Iodization reaction |
| Graphite oxide[ | Hummers method | O-containing groups | Dehydration reaction |
| Graphite[ | — | — |
| Reaction | Catalyst | Substrate | Product | Probable active site |
|---|---|---|---|---|
| Oxidation of alcohol | GO[ | Alcohol | Ketone/aldehyde | Epoxide groups |
| abGO[ | Benzylic alcohol | Benzaldehyde | Phenol hydroxyl groups | |
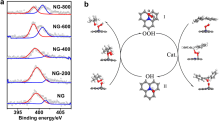
| NG-T[ | Benzylic alcohol | Benzaldehyde | Graphitic sp2-N sites | |
| BG[ | Benzylic alcohol | Benzaldehyde | B doping species (BC3) | |
| G1000[ | Benzylic alcohol | Benzaldehyde | Doped S and O atoms | |
| Oxidation of benzene | CCG[ | Benzene | Phenol | π-system of graphene and O-containing groups |
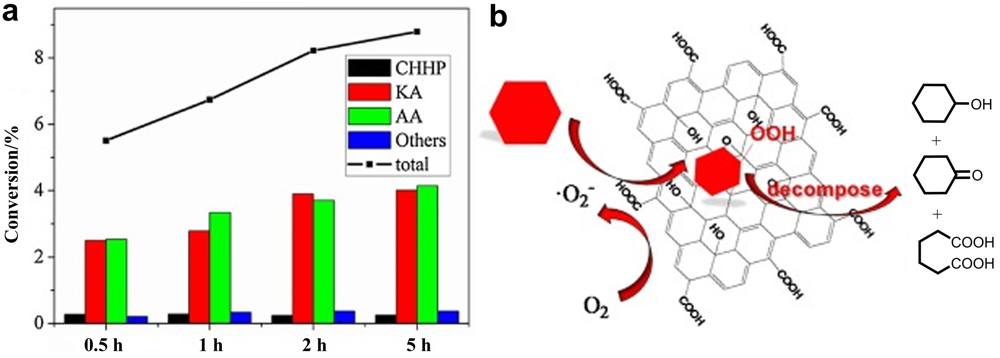
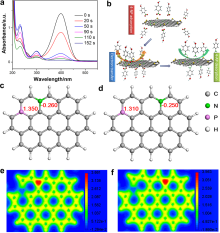
| Oxidation of cyclohexane | GO[ | Cyclohexane | Cyclohexanol Cyclohexanone Adipic acid | Carboxylic acid groups |
| Oxidation of alkyl- substituted arenes | NGO[ | Toluene | Benzoic acid | — |
| LC-N[ | Ethylbenzene | Acetophenone | C atoms adjacent to N atoms | |
| Oxidation of olefin | NG[ | Olefin | Alkylene oxide | Graphite N |
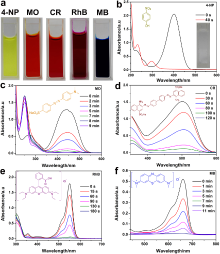
| Reduction of nitroaromatics | rGO[ | Nitrobenzene | Aniline | Edge sites |
| NG[ | Nitroaromatics | Aromatic amine | Graphitic N | |
| Reaction | Catalyst | Substrate | Product | Probable active site |
| NHG[ | Nitroaromatics | Aromatic amine | Doped N atoms | |
| NPG[ | Nitroaromatics | Aromatic amine | Doped N and P atoms | |
| Selective hydrogenation | Graphene[ | Acetylene Ethylene | Ethylene Ethane | Lewis acid-base pairs |
| Cross-coupling reaction | GO[ | Iodobenzene Benzene | Biaryl compounds | O-containing groups |
| GO[ | 1,3,5-Trimethoxy-benzene | Biaryl compounds | O-containing group, π-conjugated system and unpaired electrons | |
| Oxidative coupling of amines | Graphite oxide[ | Benzylamine | N-Benzylidene aniline | — |
| ba-GO[ | Benzylamine | N-Benzylidene aniline | The edge sites carboxyl | |
| PG[ | Benzylamine | N-Benzylidene aniline | Doped of P atoms | |
| BNHG[ | Benzylamine | N-Benzylidene aniline | Doped B and N atoms | |
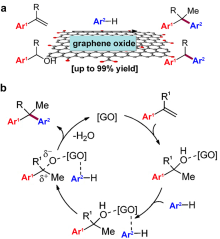
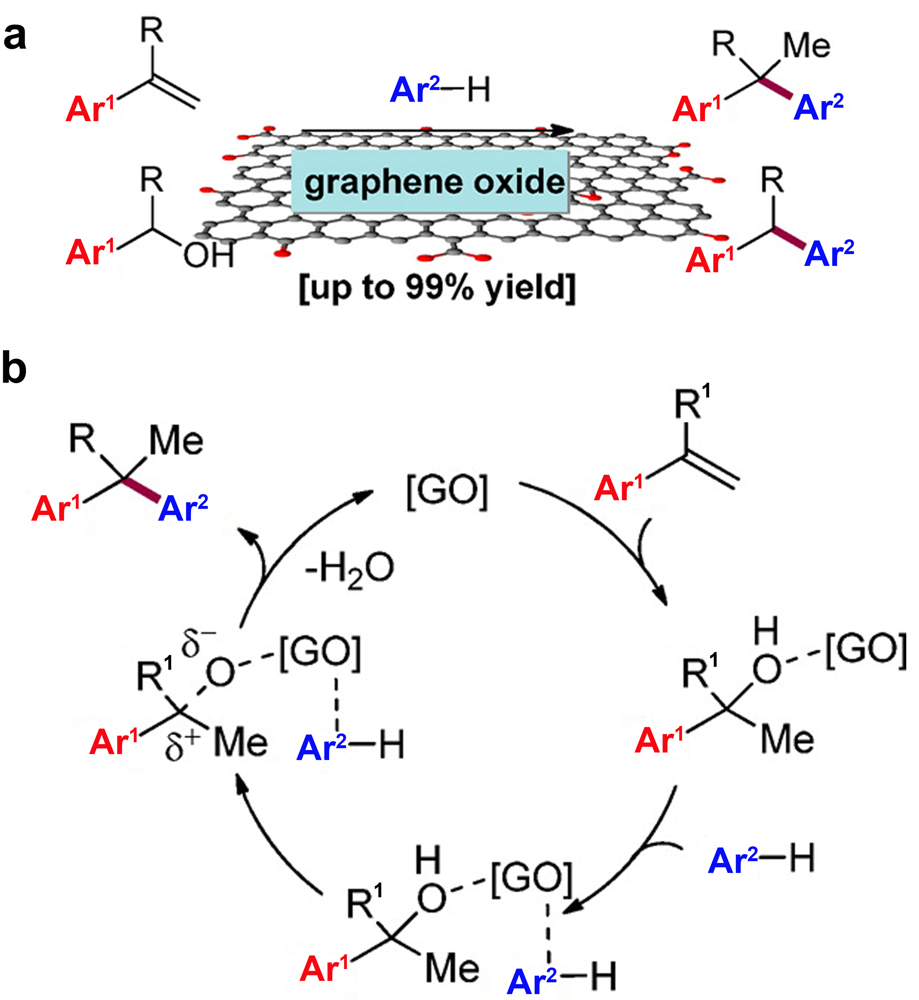
| Friedel-Crafts alkylation | GO[ | Aromatics Styrene Alcohol | Diarylalkane | O-containing groups |
| GO[ | Aldehydes Indoles | Bis(indolyl)methanes | O-containing groups | |
| GO[ | Indole Ethers | 3,3'-Bisindolylmethane | — | |
| Friedel-Crafts acylation | Graphite[ | Aromatic compounds | Acylated products | — |
| Transesterification | GR-SO3H[ | Palm oil | Biodiesel | Benzenesulfonic acid groups |
| Esterification | GO-S[ | Oleic acid | Biodiesel | Sulfonic acid groups and carboxylic acid groups |
| Claisen-Schmidt Condensation | Graphite oxide[ | Phenylacetylene Benzyl alcohol | Chalcone | — |
| Michael reaction Knoevenagel reaction | GO-DETA[ | Benzaldehyde Malononitrile (E)-Chalcone | Benzylidenemalononitrile 2-(3-oxo-1,3-diphenylpropyl)malononitrile | Primary, secondary amino and carboxyl groups |
| Hydrolysis reaction | GO[ | Cellulose | Glucose | Hydroxyl and carboxyl groups |
| N-formylation reaction | GO[ | Tetrahydroisoquinoline | Formamide | Carboxylic acid group |
| Dehydrogenation reaction | GO[ | Tetrahydroisoquinoline | Dehydrogenation products | O-containing groups |
| Iodization reaction | GO[ | Benzothiazole | Iodination product | Unpaired electrons |
| Dehydration reaction | Graphite oxide[ | Benzyl alcohol | Poly(phenylene methylene) | O-containing groups |
| Graphite[ | Ethanol Silver nitrate | Silver cyanide | — |
| Reaction | Catalyst | Substrate | Product | Probable active site |
|---|---|---|---|---|
| Oxidation of alcohol | GO[ | Alcohol | Ketone/aldehyde | Epoxide groups |
| abGO[ | Benzylic alcohol | Benzaldehyde | Phenol hydroxyl groups | |
| NG-T[ | Benzylic alcohol | Benzaldehyde | Graphitic sp2-N sites | |
| BG[ | Benzylic alcohol | Benzaldehyde | B doping species (BC3) | |
| G1000[ | Benzylic alcohol | Benzaldehyde | Doped S and O atoms | |
| Oxidation of benzene | CCG[ | Benzene | Phenol | π-system of graphene and O-containing groups |
| Oxidation of cyclohexane | GO[ | Cyclohexane | Cyclohexanol Cyclohexanone Adipic acid | Carboxylic acid groups |
| Oxidation of alkyl- substituted arenes | NGO[ | Toluene | Benzoic acid | — |
| LC-N[ | Ethylbenzene | Acetophenone | C atoms adjacent to N atoms | |
| Oxidation of olefin | NG[ | Olefin | Alkylene oxide | Graphite N |
| Reduction of nitroaromatics | rGO[ | Nitrobenzene | Aniline | Edge sites |
| NG[ | Nitroaromatics | Aromatic amine | Graphitic N | |
| Reaction | Catalyst | Substrate | Product | Probable active site |
| NHG[ | Nitroaromatics | Aromatic amine | Doped N atoms | |
| NPG[ | Nitroaromatics | Aromatic amine | Doped N and P atoms | |
| Selective hydrogenation | Graphene[ | Acetylene Ethylene | Ethylene Ethane | Lewis acid-base pairs |
| Cross-coupling reaction | GO[ | Iodobenzene Benzene | Biaryl compounds | O-containing groups |
| GO[ | 1,3,5-Trimethoxy-benzene | Biaryl compounds | O-containing group, π-conjugated system and unpaired electrons | |
| Oxidative coupling of amines | Graphite oxide[ | Benzylamine | N-Benzylidene aniline | — |
| ba-GO[ | Benzylamine | N-Benzylidene aniline | The edge sites carboxyl | |
| PG[ | Benzylamine | N-Benzylidene aniline | Doped of P atoms | |
| BNHG[ | Benzylamine | N-Benzylidene aniline | Doped B and N atoms | |
| Friedel-Crafts alkylation | GO[ | Aromatics Styrene Alcohol | Diarylalkane | O-containing groups |
| GO[ | Aldehydes Indoles | Bis(indolyl)methanes | O-containing groups | |
| GO[ | Indole Ethers | 3,3'-Bisindolylmethane | — | |
| Friedel-Crafts acylation | Graphite[ | Aromatic compounds | Acylated products | — |
| Transesterification | GR-SO3H[ | Palm oil | Biodiesel | Benzenesulfonic acid groups |
| Esterification | GO-S[ | Oleic acid | Biodiesel | Sulfonic acid groups and carboxylic acid groups |
| Claisen-Schmidt Condensation | Graphite oxide[ | Phenylacetylene Benzyl alcohol | Chalcone | — |
| Michael reaction Knoevenagel reaction | GO-DETA[ | Benzaldehyde Malononitrile (E)-Chalcone | Benzylidenemalononitrile 2-(3-oxo-1,3-diphenylpropyl)malononitrile | Primary, secondary amino and carboxyl groups |
| Hydrolysis reaction | GO[ | Cellulose | Glucose | Hydroxyl and carboxyl groups |
| N-formylation reaction | GO[ | Tetrahydroisoquinoline | Formamide | Carboxylic acid group |
| Dehydrogenation reaction | GO[ | Tetrahydroisoquinoline | Dehydrogenation products | O-containing groups |
| Iodization reaction | GO[ | Benzothiazole | Iodination product | Unpaired electrons |
| Dehydration reaction | Graphite oxide[ | Benzyl alcohol | Poly(phenylene methylene) | O-containing groups |
| Graphite[ | Ethanol Silver nitrate | Silver cyanide | — |
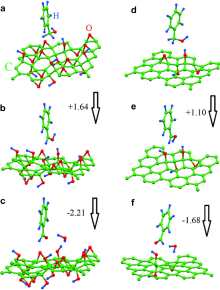





| [1] |
Chowdhury A. D.; Houben K.; Whiting G. T.; Chung S.-H.; Baldus M.; Weckhuysen B. M. Nat. Catal. 2018, 1, 23.
doi: 10.1038/s41929-017-0002-4 |
| [2] |
Bakandritsos A.; Kadam R. G.; Kumar P.; Zoppellaro G.; Medved M.; Tucek J.; Montini T.; Tomanec O.; Andryskova P.; Drahos B.; Varma R. S.; Otyepka M.; Gawande M. B.; Fornasiero P.; Zboril R. Adv. Mater. 2019, 31, 1900323.
doi: 10.1002/adma.v31.17 |
| [3] |
Zou Y. J.; Cheng H. J.; Wang H. N.; Huang R. X.; Xu Y. H.; Jiang J.; He Q.; Liu C. H.; Liu J. C.; Xiong J. M.; Yao J. N.; Huangfu X. L.; Ma J. Environ. Sci. Technol. 2020, 54, 7205.
doi: 10.1021/acs.est.0c00068 |
| [4] |
Pan L.; Xu M.-Y.; Feng L.-J.; Chen Q.; He Y.-J.; Han B.-H. Polym. Chem. 2016, 7, 2308.
doi: 10.1039/C6PY90040B |
| [5] |
Martin-Aranda R. M.; Cejka J. Top. Catal. 2010, 53, 141.
doi: 10.1007/s11244-009-9419-6 |
| [6] |
Nakagawa K. J. Jpn. Pet. Inst. 2019, 62, 53.
doi: 10.1627/jpi.62.53 |
| [7] |
Pentsak E. O.; Cherepanova V. A.; Ananikov V. P. ACS Appl. Mater. Interfaces 2017, 9, 36723.
doi: 10.1021/acsami.7b09173 |
| [8] |
Du Z. T.; Shao Z. H. Chem. Soc. Rev. 2013, 42, 1337.
doi: 10.1039/C2CS35258C |
| [9] |
Hu H. W.; Xin J. H.; Hu H.; Wang X. W.; Kong Y. Y. Appl. Catal. A-Gen. 2015, 492, 1.
doi: 10.1016/j.apcata.2014.11.041 |
| [10] |
Pandey R. K.; Prajapati V. K. Int. J. Biol. Macromol. 2018, 107, 1278.
doi: S0141-8130(17)33394-9 pmid: 29017884 |
| [11] |
Polshettiwar V.; Varma R. S. Green Chem. 2010, 12, 743.
doi: 10.1039/b921171c |
| [12] |
Su D. S.; Wen G. D.; Wu S. C.; Peng F.; Schlogl R. Angew. Chem. Int. Ed. 2017, 56, 936.
doi: 10.1002/anie.201600906 |
| [13] |
Su D. S.; Zhang J.; Frank B.; Thomas A.; Wang X. C.; Paraknowitsch J.; Schlogl R. ChemSusChem 2010, 3, 169.
doi: 10.1002/cssc.v3:2 |
| [14] |
Su D. S.; Perathoner S.; Centi G. Chem. Rev. 2013, 113, 5782.
doi: 10.1021/cr300367d |
| [15] |
Centi G.; Perathoner S.; Su D. S. Catal. Surv. Asia 2014, 18, 149.
doi: 10.1007/s10563-014-9172-0 |
| [16] |
Geim A. K.; Novoselov K. S. Nat. Mater. 2007, 6, 183.
pmid: 17330084 |
| [17] |
Novoselov K. S.; Geim A. K.; Morozov S. V.; Jiang D.; Zhang Y.; Dubonos S. V.; Grigorieva I. V.; Firsov A. A. Science 2004, 306, 666.
pmid: 15499015 |
| [18] |
Chua C. K.; Pumera M. Chem. - Eur. J. 2015, 21, 12550.
doi: 10.1002/chem.201501383 |
| [19] |
Balandin A. A.; Ghosh S.; Bao W. Z.; Calizo I.; Teweldebrhan D.; Miao F.; Lau C. N. Nano Lett. 2008, 8, 902.
doi: 10.1021/nl0731872 |
| [20] |
Stoller M. D.; Park S. J.; Zhu Y. W.; An J. H.; Ruoff R. S. Nano Lett. 2008, 8, 3498.
doi: 10.1021/nl802558y pmid: 18788793 |
| [21] |
Chua C. K.; Pumera M. Chem. Soc. Rev. 2014, 43, 291.
doi: 10.1039/C3CS60303B |
| [22] |
Huang C. S.; Li Y. J.; Wang N.; Xue Y. R.; Zuo Z. C.; Liu H. B.; Li Y. L. Chem. Rev. 2018, 118, 7744.
doi: 10.1021/acs.chemrev.8b00288 |
| [23] |
Zuo Z. C.; Li Y. L. Joule 2019, 3, 899.
doi: 10.1016/j.joule.2019.01.016 |
| [24] |
Du Y. C.; Zhou W. D.; Gao J.; Pan X. Y.; Li Y. L. Acc. Chem. Res. 2020, 53, 459.
doi: 10.1021/acs.accounts.9b00558 |
| [25] |
Hui L.; Xue Y. R.; Yu H. D.; Liu Y. X.; Fang Y.; Xing C. Y.; Huang B. L.; Li Y. L. J. Am. Chem. Soc. 2019, 141, 10677.
doi: 10.1021/jacs.9b03004 |
| [26] |
Xue Y. R.; Huang B. L.; Yi Y. P.; Guo Y.; Zuo Z. C.; Li Y. J.; Jia Z. Y.; Liu H. B.; Li Y. L. Nat. Commun. 2018, 9, 1460.
doi: 10.1038/s41467-018-03896-4 |
| [27] |
Li L.; Zuo Z. C.; Wang F.; Gao J. C.; Cao A. M.; He F.; Li Y. L. Adv. Mater. 2020, 32, 2000140.
doi: 10.1002/adma.v32.14 |
| [28] |
Roy A. S.; Poulose A. C.; Bakandritsos A.; Varma R. S.; Otyepka M. Appl. Mater. Today 2021, 23, 101053.
|
| [29] |
Li X. T.; Wang J.; Duan X. G.; Li Y.; Fan X. B.; Zhang G. L.; Zhang F. B.; Peng W. C. ACS Catal. 2021, 11, 4848.
doi: 10.1021/acscatal.0c05089 |
| [30] |
Park M.; Lee J.; Kim B. S. Nanoscale 2021, 13, 10143.
doi: 10.1039/D1NR02025K |
| [31] |
Ahmad M. S.; Nishina Y. Nanoscale 2020, 12, 12210.
doi: 10.1039/D0NR02984J |
| [32] |
Wang Z. Y.; Pu Y.; Wang D.; Wang J. X.; Chen J. F. Front. Chem. Sci. Eng. 2018, 12, 855.
doi: 10.1007/s11705-018-1722-y |
| [33] |
Liu J. Q.; Tang J. G.; Gooding J. J. J. Mater. Chem. 2012, 22, 12435.
doi: 10.1039/c2jm31218b |
| [34] |
Daelemans B.; Bilbao N.; Dehaen W.; De Feyter S. Chem. Soc. Rev. 2021, 50, 2280.
doi: 10.1039/d0cs01294g pmid: 33404567 |
| [35] |
Srivastava S. K.; Pionteck J. J. Nanosci. Nanotechnol. 2015, 15, 1984.
pmid: 26413611 |
| [36] |
Dreyer D. R.; Todd A. D.; Bielawski C. W. Chem. Soc. Rev. 2014, 43, 5288.
doi: 10.1039/C4CS00060A |
| [37] |
Wang X. W.; Sun G. Z.; Routh P.; Kim D. H.; Huang W.; Chen P. Chem. Soc. Rev. 2014, 43, 7067.
doi: 10.1039/C4CS00141A |
| [38] |
Dimiev A. M.; Tour J. M. ACS Nano 2014, 8, 3060.
doi: 10.1021/nn500606a pmid: 24568241 |
| [39] |
Rosillo-Lopez M.; Lee T. J.; Bella M.; Hart M.; Salzmann C. G. RSC Adv. 2015, 5, 104198.
doi: 10.1039/C5RA23209K |
| [40] |
Wang C. I.; Periasamy A. P.; Chang H. T. Anal. Chem. 2013, 85, 3263.
doi: 10.1021/ac303613d |
| [41] |
Sun L. Chin. J. Chem. Eng. 2019, 27, 2251.
doi: 10.1016/j.cjche.2019.05.003 |
| [42] |
Dreyer D. R.; Park S.; Bielawski C. W.; Ruoff R. S. Chem. Soc. Rev. 2010, 39, 228.
doi: 10.1039/B917103G |
| [43] |
Lerf A.; He H.; Forster M. Phys. Chem. B 1998, 102, 4477.
doi: 10.1021/jp9731821 |
| [44] |
Yang J. H.; Yang D.; Tang P.; Ma D. Acta Phys.-Chim. Sin. 2016, 32, 75. (in Chinese)
doi: 10.3866/PKU.WHXB201512153 |
|
( 杨敬贺, 杨朵, 唐沛, 马丁, 物理化学学报, 2016, 32, 75.)
|
|
| [45] |
Loh K. P.; Bao Q. L.; Eda G.; Chhowalla M. Nat. Chem. 2010, 2, 1015.
doi: 10.1038/nchem.907 |
| [46] |
Wan W. B.; Li L. L.; Zhao Z. B.; Hu H.; Hao X. J.; Winkler D. A.; Xi L. C.; Hughes T. C.; Qiu J. S. Adv. Funct. Mater. 2014, 24, 4915.
doi: 10.1002/adfm.201303815 |
| [47] |
Eigler S.; Hu Y. C.; Ishii Y.; Hirsch A. Nanoscale 2013, 5, 12136.
doi: 10.1039/c3nr04332k |
| [48] |
Wang Y.; Li S. S.; Yang H. Y.; Luo J. RSC Adv. 2020, 10, 15328.
doi: 10.1039/D0RA01068E |
| [49] |
Su C. L.; Loh K. P. Acc. Chem. Res. 2013, 46, 2275.
doi: 10.1021/ar300118v |
| [50] |
Feng J. L.; Ye Y. Q.; Xiao M.; Wu G.; Ke Y. Chem. Pap. 2020, 74, 3767.
doi: 10.1007/s11696-020-01196-0 |
| [51] |
Larciprete R.; Fabris S.; Sun T.; Lacovig P.; Baraldi A.; Lizzit S. J. Am. Chem. Soc. 2011, 133, 17315.
doi: 10.1021/ja205168x pmid: 21846143 |
| [52] |
Xu C.; Yuan R. S.; Wang X. New Carbon Mater. 2014, 29, 61.
doi: 10.1016/S1872-5805(14)60126-8 |
| [53] |
Moon I. K.; Lee J.; Ruoff R. S.; Lee H. Nat. Commun. 2010, 1, 1.
|
| [54] |
Navalon S.; Dhakshinamoorthy A.; Alvaro M.; Garcia H. Chem. Rev. 2014, 114, 6179.
doi: 10.1021/cr4007347 pmid: 24867457 |
| [55] |
Yang J. H.; Sun G.; Gao Y. J.; Zhao H. B.; Tang P.; Tan J.; Lu A. H.; Ma D. Energy Environ. Sci. 2013, 6, 793.
doi: 10.1039/c3ee23623d |
| [56] |
Wang Z.; Pu Y.; Wang D.; Wang J.-X.; Chen J.-F. Front. Chem. Sci. Eng. 2018, 12, 855.
doi: 10.1007/s11705-018-1722-y |
| [57] |
Azlouk M.; Durmaz M.; Zor E.; Bingol H. Mater. Chem. Phys. 2020, 239, 122298.
doi: 10.1016/j.matchemphys.2019.122298 |
| [58] |
Liu H. T.; Liu Y. Q.; Zhu D. B. J. Mater. Chem. 2011, 21, 3335.
doi: 10.1039/C0JM02922J |
| [59] |
Zhang D. Y.; Lei L. Y.; Shang Y. H. Chem. Ind. Eng. Prog. 2016, 35, 831. (in Chinese)
|
|
( 张德懿, 雷龙艳, 尚永花, 化工进展, 2016, 35, 831.)
|
|
| [60] |
Zheng Y.; Jiao Y.; Jaroniec M.; Jin Y. G.; Qiao S. Z. Small 2012, 8, 3550.
doi: 10.1002/smll.201200861 pmid: 22893586 |
| [61] |
Feng L. Y.; Qin Z. Y.; Huang Y. J.; Peng K. S.; Wang F.; Yan Y. Y.; Chen Y. G. Sci. Total Environ. 2020, 698, 134239.
doi: 10.1016/j.scitotenv.2019.134239 |
| [62] |
Li X. H.; Antonietti M. Angew. Chem. Int. Ed. 2013, 52, 4572.
doi: 10.1002/anie.201209320 |
| [63] |
Patel M. A.; Luo F. X.; Khoshi M. R.; Rabie E.; Zhang Q.; Flach C. R.; Mendelsohn R.; Garfunkel E.; Szostak M.; He H. X. ACS Nano 2016, 10, 2305.
doi: 10.1021/acsnano.5b07054 |
| [64] |
Kong X.-K.; Chen C.-L.; Chen Q.-W. Chem. Soc. Rev. 2014, 43, 2841.
doi: 10.1039/C3CS60401B |
| [65] |
Guo X. L.; Qi W.; Liu W.; Yan P. Q.; Li F.; Liang C. H.; Su D. S. ACS Catal. 2017, 7, 1424.
doi: 10.1021/acscatal.6b02936 |
| [66] |
Dreyer D. R.; Jia H.-P.; Bielawski C. W. Angew. Chem., Int. Ed. 2010, 49, 6813.
|
| [67] |
Boukhvalov D. W.; Dreyer D. R.; Bielawski C. W.; Son Y. W. ChemCatChem 2012, 4, 1844.
doi: 10.1002/cctc.v4.11 |
| [68] |
Zhu S. H.; Cen Y. L.; Yang M. A.; Guo J.; Chen C. M.; Wang J. G.; Fan W. B. Appl. Catal., B-Environ. 2017, 211, 89.
doi: 10.1016/j.apcatb.2017.04.035 |
| [69] |
Long J. L.; Xie X. Q.; Xu J.; Gu Q.; Chen L. M.; Wang X. X. ACS Catal. 2012, 2, 622.
doi: 10.1021/cs3000396 |
| [70] |
Cheng W. J.; Liu X. T.; Li N.; Han J. T.; Li S. M.; Yu S. S. RSC Adv. 2018, 8, 11222.
doi: 10.1039/C8RA00290H |
| [71] |
Zhu S. H.; Chen Y. Y.; Gao X. Q.; Lv Z. X.; He Y.; Wang J. G.; Fan W. B. Catal. Sci. Technol. 2020, 10, 2786.
doi: 10.1039/C9CY02476J |
| [72] |
Xiao Y. P.; Liu J. C.; Xie K. H.; Wang W. B.; Fang Y. X. Mol. Catal. 2017, 431, 1.
|
| [73] |
Heidari M.; Sedrpoushan A.; Mohannazadeh F. Org. Process Res. Dev. 2017, 21, 641.
doi: 10.1021/acs.oprd.7b00056 |
| [74] |
Gao Y. J.; Hu G.; Zhong J.; Shi Z. J.; Zhu Y. S.; Su D. S.; Wang J. G.; Bao X. H.; Ma D. Angew. Chem. Int. Ed. 2013, 52, 2109.
doi: 10.1002/anie.v52.7 |
| [75] |
Li W. J.; Gao Y. J.; Chen W. L.; Tang P.; Li W. Z.; Shi Z. J.; Su D. S.; Wang J. G.; Ma D. ACS Catal. 2014, 4, 1261.
doi: 10.1021/cs500062s |
| [76] |
Gao Y. J.; Ma D.; Wang C. L.; Guan J.; Bao X. H. Chem. Commun. 2011, 47, 2432.
doi: 10.1039/C0CC04420B |
| [77] |
Yang F.; Chi C.; Wang C. X.; Wang Y.; Li Y. F. Green Chem. 2016, 18, 4254.
doi: 10.1039/C6GC00222F |
| [78] |
He Z. L.; Liu J.; Wang Q. J.; Zhao W.; Wen Z. P.; Chen J.; Manoj D.; Xie C. Y.; Xi J. B.; Yu J. X.; Tang C. Y.; Bai Z. W.; Wang S. J. Catal. 2019, 377, 199.
doi: 10.1016/j.jcat.2019.07.017 |
| [79] |
Xi J. B.; Wang Q. J.; Liu J.; Huan L.; He Z. L.; Qiu Y.; Zhang J.; Tang C. Y.; Xiao J.; Wang S. J. Catal. 2018, 359, 233.
doi: 10.1016/j.jcat.2018.01.003 |
| [80] |
Primo A.; Neatu F.; Florea M.; Parvulescu V.; Garcia H. Nat. Commun. 2014, 5, 5291.
doi: 10.1038/ncomms6291 pmid: 25342228 |
| [81] |
Gao Y. J.; Tang P.; Zhou H.; Zhang W.; Yang H. J.; Yan N.; Hu G.; Mei D. H.; Wang J. G.; Ma D. Angew. Chem., Int. Ed. 2016, 55, 3124.
doi: 10.1002/anie.201510081 |
| [82] |
Fang J. X.; Peng Z. Y.; Yang Y.; Wang J. W.; Guo J. Y.; Gong H. Asian J. Org. Chem. 2018, 7, 355.
doi: 10.1002/ajoc.v7.2 |
| [83] |
Huang H.; Huang J.; Liu Y. M.; He H. Y.; Cao Y.; Fan K. N. Green Chem. 2012, 14, 930.
doi: 10.1039/c2gc16681j |
| [84] |
Su C. L.; Acik M.; Takai K.; Lu J.; Hao S. J.; Zheng Y.; Wu P. P.; Bao Q. L.; Enoki T.; Chabal Y. J.; Loh K. P. Nat. Commun. 2012, 3, 1.
|
| [85] |
Yang F.; Fan X. X.; Wang C. X.; Yang W.; Hou L. Q.; Xu X. W.; Feng A. D.; Dong S.; Chen K.; Wang Y.; Li Y. F. Carbon 2017, 121, 443.
doi: 10.1016/j.carbon.2017.05.101 |
| [86] |
Hu F.; Patel M.; Luo F. X.; Flach C.; Mendelsohn R.; Garfunkel E.; He H. X.; Szostak M. J. Am. Chem. Soc. 2015, 137, 14473.
doi: 10.1021/jacs.5b09636 |
| [87] |
Wang Y. H.; Sang R.; Zheng Y.; Guo L.; Guan M.; Wu Y. Catal. Commun. 2017, 89, 138.
doi: 10.1016/j.catcom.2016.09.027 |
| [88] |
Peng X. J.; Zen Y.; Liu Q.; Liu L. X.; Wang H. S. Org. Chem. Front. 2019, 6, 3615.
doi: 10.1039/C9QO00926D |
| [89] |
Kodomari M.; Suzuki Y.; Yoshida K. Chem. Commun. 1997, 1567.
|
| [90] |
Nongbe M. C.; Ekou T.; Ekou L.; Yao K. B.; Le Grognec E.; Felpin F. X. Renew. Energ. 2017, 106, 135.
doi: 10.1016/j.renene.2017.01.024 |
| [91] |
Zhang H. L.; Luo X.; Shi K. Q.; Wu T.; He F.; Zhou S. B.; Chen G. Z.; Peng C. ChemSusChem 2017, 10, 3352.
doi: 10.1002/cssc.201700950 |
| [92] |
Jia H. P.; Dreyer D. R.; Bielawski C. W. Adv. Synth. Catal. 2011, 353, 528.
doi: 10.1002/adsc.201000748 |
| [93] |
Yang A. W.; Li J. J.; Zhang C.; Zhang W. Q.; Ma N. Appl. Surf. Sci. 2015, 346, 443.
doi: 10.1016/j.apsusc.2015.04.033 |
| [94] |
Zhao X. C.; Wang J.; Chen C. M.; Huang Y. Q.; Wang A. Q.; Zhang T. Chem. Commun. 2014, 50, 3439.
doi: 10.1039/c3cc49634a |
| [95] |
Ma J.; Zhang J. Y.; Zhou X.; Wang J. W.; Gong H. J. Iran. Chem. Soc. 2018, 15, 2851.
doi: 10.1007/s13738-018-1471-3 |
| [96] |
Zhang J. Y.; Chen S. Y.; Chen F. F.; Xu W. S.; Deng G. J.; Gong H. Adv. Synth. Catal. 2017, 359, 2358.
doi: 10.1002/adsc.v359.14 |
| [97] |
Zhang J. Y.; Li S. G.; Deng G. J.; Gong H. ChemCatChem 2018, 10, 376.
doi: 10.1002/cctc.v10.2 |
| [98] |
Dreyer D. R.; Jarvis K. A.; Ferreira P. J.; Bielawski C. W. Macromolecules 2011, 44, 7659.
doi: 10.1021/ma201306x |
| [99] |
Xiao D.; Wang W. C.; Gai Y. Z.; Zhao Y. Sci. Rep. 2018, 8, 1750.
doi: 10.1038/s41598-018-20238-y |
| [100] |
Tang P.; Hu G.; Li M. Z.; Ma D. ACS Catal. 2016, 6, 6948.
doi: 10.1021/acscatal.6b01668 |
| [101] |
Wen G. D.; Gu Q. Q.; Liu Y. F.; Schlogl R.; Wang C. X.; Tian Z. J.; Su D. S. Angew. Chem. Int. Ed. 2018, 57, 16898.
doi: 10.1002/anie.v57.51 |
| [102] |
Chen B.; Wang L. Y.; Gao S. ACS Catal. 2015, 5, 5851.
doi: 10.1021/acscatal.5b01479 |
| [103] |
Mukherjee A.; Nerush A.; Leitus G.; Shimon L. J. W.; Ben David Y.; Jalapa N. A. E.; Milstein D. J. Am. Chem. Soc. 2016, 138, 4298.
doi: 10.1021/jacs.5b13519 pmid: 26924231 |
| [1] | 宁聪聪, 杨倩, 毛阿敏, 唐梓嘉, 金燕, 胡宝山. 石墨烯纳米带的可控制备研究进展[J]. 化学学报, 2023, 81(4): 406-419. |
| [2] | 刘稳, 王昱捷, 杨慧琴, 李成杰, 吴娜, 颜洋. 离子液体非共价诱导制备碳纳米管/石墨烯集流体用于钠金属负极[J]. 化学学报, 2023, 81(10): 1379-1386. |
| [3] | 闫绍兵, 焦龙, 何传新, 江海龙. ZIF-67/石墨烯复合物衍生的氮掺杂碳限域Co纳米颗粒用于高效电催化氧还原[J]. 化学学报, 2022, 80(8): 1084-1090. |
| [4] | 王旭生, 杨胥, 陈春辉, 李红芳, 黄远标, 曹荣. 石墨烯量子点/铁基金属-有机骨架复合材料高效光催化二氧化碳还原※[J]. 化学学报, 2022, 80(1): 22-28. |
| [5] | 翟耀, 辛国祥, 王佳琦, 张邦文, 宋金玲, 刘晓旭. 微波辅助合成具有优异电化学性能的rGO/CeO2超级电容器电极材料[J]. 化学学报, 2021, 79(9): 1129-1137. |
| [6] | 刘长安, 洪士博, 李蓓. 石墨烯在甘油/尿素剥离液中的稳定行为的分子动力学模拟研究[J]. 化学学报, 2021, 79(4): 530-538. |
| [7] | 曾誉, 吕品, 蔡跃进, 高福杰, 卓欧, 吴强, 杨立军, 王喜章, 胡征. 分级结构碳纳米笼高效催化苄胺氧化偶联制N-苄烯丁胺[J]. 化学学报, 2021, 79(4): 539-544. |
| [8] | 岳华, 马光辉. 基于石墨烯独特生物界面效应的功能化载体研究进展[J]. 化学学报, 2021, 79(10): 1244-1256. |
| [9] | 马明昊, 徐明, 刘思金. 氧化石墨烯的表面化学修饰及纳米-生物界面作用机理[J]. 化学学报, 2020, 78(9): 877-887. |
| [10] | 李海梅, 罗华健, 肖琦, 杨立云, 黄珊, 刘义. 手性石墨烯量子点与DNA相互作用及其机制研究[J]. 化学学报, 2020, 78(6): 577-586. |
| [11] | 赵雅婧, 谢亮, 马兰超, 贺军辉. 聚二甲基硅氧烷封装石墨烯基柔性红外探测器的制备及其应用[J]. 化学学报, 2020, 78(2): 161-169. |
| [12] | 宋光捷, 武调弟, 刘福鑫, 张彬雁, 刘秀辉. 壳聚糖/氮掺杂还原氧化石墨烯修饰电极对黄嘌呤的检测及尿酸抑制的研究[J]. 化学学报, 2020, 78(1): 82-88. |
| [13] | 郭宇, 李燕瑞, 王成名, 龙冉, 熊宇杰. TiO2/石墨烯复合材料的光生电荷分离调控与光催化产氢性能研究[J]. 化学学报, 2019, 77(6): 520-524. |
| [14] | 卢静荷, 谭淑珍, 朱雨清, 李伟, 陈天啸, 王瑶, 刘陈. 荧光核酸适配体功能化氧化石墨烯生物传感器用于快速检测氯霉素[J]. 化学学报, 2019, 77(3): 253-256. |
| [15] | 丁锐, 陈思, 吕静, 桂泰江, 王晓, 赵晓栋, 刘杰, 李秉钧, 宋立英, 李伟华. 石墨烯在防腐薄膜和有机防腐涂层领域的理论和应用研究综述[J]. 化学学报, 2019, 77(11): 1140-1155. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||