化学学报 ›› 2024, Vol. 82 ›› Issue (4): 396-408.DOI: 10.6023/A23120529 上一篇 下一篇
研究论文
雷雅茹a, 熊廷楷a, 于湘涛b, 黄秀兵c, 唐晓龙a,*( ), 易红宏a, 周远松a, 赵顺征a, 孙龙a, 高凤雨a,*(
), 易红宏a, 周远松a, 赵顺征a, 孙龙a, 高凤雨a,*( )
)
投稿日期:2023-12-11
发布日期:2024-03-05
Yaru Leia, Tingkai Xionga, Xiangtao Yub, Xiubing Huangc, Xiaolong Tanga,*( ), Honghong Yia, Yuansong Zhoua, Shunzheng Zhaoa, Long Suna, Fengyu Gaoa,*(
), Honghong Yia, Yuansong Zhoua, Shunzheng Zhaoa, Long Suna, Fengyu Gaoa,*( )
)
Received:2023-12-11
Published:2024-03-05
Contact:
* E-mail: 文章分享
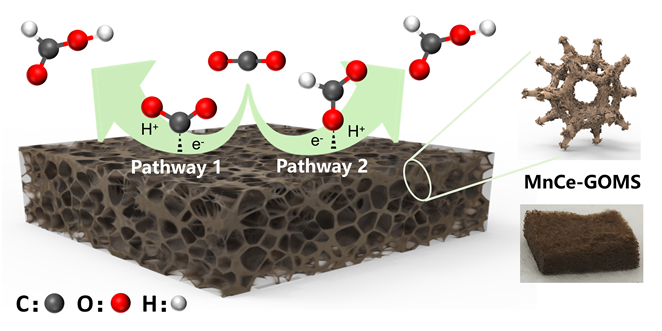
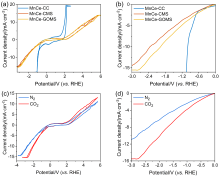
本研究提出了一种尚未见报道的CO2还原电催化剂及其构造, 由MnCe作为活性位点, 三聚氰胺泡沫(MS)作为载体前驱体的新型电极材料——MnCe-CMS(碳化MS)和MnCe-GOMS(氧化石墨烯活化MS), 用于电催化CO2还原研究. 结果发现, MnCe-MS具有较宽的电位范围(–0.2~–3 V vs. RHE)及较好的产甲酸能力. 对比以常用的碳布(CC)为载体的MnCe-CC, MnCe-CMS和MnCe-GOMS的甲酸生成速率分别提高到2.3、2.8倍, 法拉第效率分别提高到2.3、2.5倍(MnCe-CC的最佳电位–0.4 V条件下), 并且MnCe-GOMS在–0.6 V表现出最佳甲酸法拉第效率(75.72%). 这归因于MS材料丰富的孔隙结构、较大的电化学表面积、易形成碳缺陷的特点, 分析表明GO的掺入可以进一步增大这些优势; 此外, 在Mn、Ce共同作用下, 有效促进电子传输、抑制析氢竞争反应、形成氧空位, 有利于CO2的吸附、活化与转化, 从而促进甲酸生成.
雷雅茹, 熊廷楷, 于湘涛, 黄秀兵, 唐晓龙, 易红宏, 周远松, 赵顺征, 孙龙, 高凤雨. 新型多孔三聚氰胺负载MnCe用于高选择性电催化CO2产甲酸[J]. 化学学报, 2024, 82(4): 396-408.
Yaru Lei, Tingkai Xiong, Xiangtao Yu, Xiubing Huang, Xiaolong Tang, Honghong Yi, Yuansong Zhou, Shunzheng Zhao, Long Sun, Fengyu Gao. Novel Porous Melamine Foam Loaded with MnCe for Highly Selective Electrocatalytic CO2 to Formic Acid[J]. Acta Chimica Sinica, 2024, 82(4): 396-408.
| Catalysts | Onset potential (V vs. RHE) | exit potential (V vs. RHE) | Selection of experimental sites (V vs. RHE) | Maximum yield rate/ (μg•h–1•cm–2) | Optimum FEf/% |
|---|---|---|---|---|---|
| MnCe-GOMS | –0.06 | –2.99 | –0.2, –0.4, –0,5, –0.6, –0.8, –1.0, –2.0, –2.5 | 746.92 (–0.8 V) | 75.72 (–0.6 V) |
| MnCe-CMS | –0.13 | –3.1 | –0.2, –0.4, –1.0, –2.0, –3.0 | 470.89 (–3.0 V) | 63.04 (–0.4 V) |
| MnCe-CC | 0.58 | –1.06 | 0.0, –0.2, –0.4, –0.8, –1.0 | 78.69 (–0.4 V) | 64.14 (0 V) |
| MnCe-CF | 0.65 | –0.23 | 0.0, –0.1, –0.2 | 489.62 (–0.2 V) | 11.67 (0 V) |
| MnCe-NF | 0.43 | –0.74 | –0.4, –0.5, –0.6, –0.7 | 542.69 (–0.4 V) | 41.47 (–0.4 V) |
| Catalysts | Onset potential (V vs. RHE) | exit potential (V vs. RHE) | Selection of experimental sites (V vs. RHE) | Maximum yield rate/ (μg•h–1•cm–2) | Optimum FEf/% |
|---|---|---|---|---|---|
| MnCe-GOMS | –0.06 | –2.99 | –0.2, –0.4, –0,5, –0.6, –0.8, –1.0, –2.0, –2.5 | 746.92 (–0.8 V) | 75.72 (–0.6 V) |
| MnCe-CMS | –0.13 | –3.1 | –0.2, –0.4, –1.0, –2.0, –3.0 | 470.89 (–3.0 V) | 63.04 (–0.4 V) |
| MnCe-CC | 0.58 | –1.06 | 0.0, –0.2, –0.4, –0.8, –1.0 | 78.69 (–0.4 V) | 64.14 (0 V) |
| MnCe-CF | 0.65 | –0.23 | 0.0, –0.1, –0.2 | 489.62 (–0.2 V) | 11.67 (0 V) |
| MnCe-NF | 0.43 | –0.74 | –0.4, –0.5, –0.6, –0.7 | 542.69 (–0.4 V) | 41.47 (–0.4 V) |




| Adsorption site types | Weak adsorption | Medium adsorption | Strong adsorption | Amount | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1# (30~180 ℃) | 2# (180~350 ℃) | 3# (350~430 ℃) | 4# (400~600 ℃) | 5# (600~800 ℃) | |||||
| MnCe-CMS | Area | 34.45 | 14.98 | 3.10 | 35.42 | 95.67 | 183.62 | ||
| Proportion% | 18.76 | 8.16 | 1.69 | 19.29 | 52.10 | 100 | |||
| MnCe-GOMS | Area | 9.28 | 28.01 | 94.40 | 101.29 | 24.48 | 248.18 | ||
| Proportion% | 3.60 | 10.88 | 36.67 | 39.34 | 9.51 | 100 | |||
| Adsorption site types | Weak adsorption | Medium adsorption | Strong adsorption | Amount | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1# (30~180 ℃) | 2# (180~350 ℃) | 3# (350~430 ℃) | 4# (400~600 ℃) | 5# (600~800 ℃) | |||||
| MnCe-CMS | Area | 34.45 | 14.98 | 3.10 | 35.42 | 95.67 | 183.62 | ||
| Proportion% | 18.76 | 8.16 | 1.69 | 19.29 | 52.10 | 100 | |||
| MnCe-GOMS | Area | 9.28 | 28.01 | 94.40 | 101.29 | 24.48 | 248.18 | ||
| Proportion% | 3.60 | 10.88 | 36.67 | 39.34 | 9.51 | 100 | |||


| Catalysts | Element content/% | Atomic ratio/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | N | O | Mn | Ce | Mn3+/Mn | Ce3+/Ce | Oα/O | Oβ/O | ||
| MnCe-CC | 60.10 | 0.37 | 30.33 | 3.60 | 5.57 | 30.75 | 9.30 | 46.81 | 53.19 | |
| MnCe-CMS | 38.00 | 1.20 | 46.55 | 8.67 | 5.58 | 32.29 | 11.09 | 37.69 | 26.81 | |
| MnCe-GOMS | 55.87 | 4.49 | 33.22 | 4.68 | 4.49 | 40.45 | 24.92 | 48.62 | 11.76 | |
| Catalysts | Element content/% | Atomic ratio/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | N | O | Mn | Ce | Mn3+/Mn | Ce3+/Ce | Oα/O | Oβ/O | ||
| MnCe-CC | 60.10 | 0.37 | 30.33 | 3.60 | 5.57 | 30.75 | 9.30 | 46.81 | 53.19 | |
| MnCe-CMS | 38.00 | 1.20 | 46.55 | 8.67 | 5.58 | 32.29 | 11.09 | 37.69 | 26.81 | |
| MnCe-GOMS | 55.87 | 4.49 | 33.22 | 4.68 | 4.49 | 40.45 | 24.92 | 48.62 | 11.76 | |
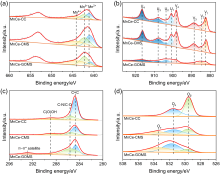


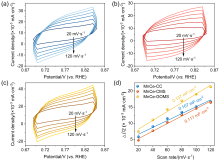

| Parameters | MnCe-CC | MnCe-CMS | MnCe-GOMS |
|---|---|---|---|
| b/(mV•dec–1) | –396 | –1750 | –1169 |
| i0/(mA•cm–2) | 0.042 | 0.783 | 0.575 |
| k0/(cm•s–1) | 7.0×10–6 | 1.23×10–5 | 9.1×10–6 |
| α | –0.149 | –0.034 | –0.051 |
| Parameters | MnCe-CC | MnCe-CMS | MnCe-GOMS |
|---|---|---|---|
| b/(mV•dec–1) | –396 | –1750 | –1169 |
| i0/(mA•cm–2) | 0.042 | 0.783 | 0.575 |
| k0/(cm•s–1) | 7.0×10–6 | 1.23×10–5 | 9.1×10–6 |
| α | –0.149 | –0.034 | –0.051 |
| Catalysts | Carrier | Advantages | Disadvantages | Potentiostatic properties |
|---|---|---|---|---|
| MnCe-CMS | Melamine foam (MS) | High FE, high yield and low cost | Low conductivity and poor stability | Wider potential range (–0.2~–3 V) |
| MnCe-GOMS | ||||
| MnCe-CC | Carbon cloth (CC) | Higher FE | Low yield | Narrower potential range (0~–1 V) |
| MnCe-CF | Metal foams | High conductivity and higher yield | Low FE, severe HER competitive response and poor reproducibility | Narrow potential range (<–0.7 V) |
| MnCe-NF |
| Catalysts | Carrier | Advantages | Disadvantages | Potentiostatic properties |
|---|---|---|---|---|
| MnCe-CMS | Melamine foam (MS) | High FE, high yield and low cost | Low conductivity and poor stability | Wider potential range (–0.2~–3 V) |
| MnCe-GOMS | ||||
| MnCe-CC | Carbon cloth (CC) | Higher FE | Low yield | Narrower potential range (0~–1 V) |
| MnCe-CF | Metal foams | High conductivity and higher yield | Low FE, severe HER competitive response and poor reproducibility | Narrow potential range (<–0.7 V) |
| MnCe-NF |




| [1] |
(a) Lei, Y. R.; Wang, Z.; Bao, A.; Tang, X. L.; Huang, X. B.; Yi, H. H.; Zhao, S. Z.; Sun, T.; Wang, J. Y.; Gao, F. Y. Chem. Eng. J. 2022, 453, 139663.
doi: 10.1016/j.cej.2022.139663 |
|
(b) Jiang, Y.; Li, G.; Chen, Q.; Xu, Z.; Lin, S.; Guo, G. Acta Chim. Sinica 2022, 80, 703. (in Chinese)
doi: 10.6023/A22010012 |
|
|
(蒋银龙, 李国超, 陈青松, 徐忠宁, 林姗姗, 郭国聪, 化学学报, 2022, 80, 703).
doi: 10.6023/A22010012 |
|
|
(c) Guo, Q.; Fu, J. L.; Zhang, C. Y.; Cai, C. Y.; Wang, C.; Zhou, L. H.; Xu, R. B.; Wang, M. Y. J. Electrochem. 2021, 27, 449. (in Chinese)
|
|
|
(郭茜, 富佳龙, 张成燕, 蔡超越, 王城, 周丽华, 许瑞波, 王明艳, 电化学, 2021, 27, 449.)
doi: 10.13208/j.electrochem.200513 |
|
| [2] |
(a) Yang, Y.; Zhang, H. Y.; Wang, Y.; Shao, L. H.; Fang, L.; Dong, H.; Lu, M.; Dong, L. Z.; Lan, Y. Q.; Zhang, F. M. Adv. Mater. 2023, 35, 2304170.
doi: 10.1002/adma.v35.40 |
|
(b) Lan, B. Y.; Shi, H. F. Acta Phys. Chim. Sinica 2014, 30, 2177. (in Chinese)
|
|
|
(蓝奔月, 史海峰, 物理化学学报, 2014, 30, 2177).
|
|
| [3] |
(a) Jiang, Q. Chin. J. Appl. Chem. 2001, 18, 536. (in Chinese)
|
|
(江琦, 应用化学, 2001, 18, 536.)
|
|
|
(b) Song, Z. J.; Liu, J.; Bai, Y.; Li, J. Y.; Peng, J. J. Chin. J. Org. Chem. 2023, 43, 2068. (in Chinese)
doi: 10.6023/cjoc202210024 |
|
|
(宋姿洁, 刘俊, 白赢, 厉嘉云, 彭家建, 有机化学, 2023, 43, 2068).
|
|
|
(c) Cui, G. Q.; Hu, Y. Y.; Lou, Y. J.; Zhou, M. X.; Li, Y. M.; Wang, Y. J.; Jiang, G. Y.; Xu, C. M. Acta Chim. Sinica 2023, 81, 1081. (in Chinese)
doi: 10.6023/A23040126 |
|
|
(崔国庆, 胡溢玚, 娄颖洁, 周明霞, 李宇明, 王雅君, 姜桂元, 徐春明, 化学学报, 2023, 81, 1081).
doi: 10.6023/A23040126 |
|
|
(d) Liu, C. H.; Guo, X. M.; Zhong, C. L.; Li, L.; Hua, Y. X.; Mao, D. S.; Lu, G. Z. Chin. J. Inorg. Chem. 2016, 32, 1405. (in Chinese)
|
|
|
(刘超恒, 郭晓明, 钟成林, 李亮, 华玉喜, 毛东森, 卢冠忠, 无机化学学报, 2016, 32, 1405.)
|
|
| [4] |
Zhang, J. F.; Wang, L.; Sun, Y. 2016, 79, 958. (in Chinese)
|
|
(张佳凤, 王黎, 孙杨, 化学通报, 2016, 79, 958.)
|
|
| [5] |
(a) Zhang, W.; Yi, H.; Ma, L.; Zhu, G.; Zhong, J. Adv. Sci. 2017, 5, 1700275.
doi: 10.1002/advs.v5.1 |
|
(b) Cheng, Y.; Yang, S. Z.; Jiang, S. P.; Wang, S. Y. Small Methods 2019, 3, 1800440.
doi: 10.1002/smtd.v3.9 |
|
| [6] |
Mori, K.; Futamura, Y.; Masuda, S.; Kobayashi, H.; Yamashita, H. Nat. Commun. 2019, 10, 4094.
doi: 10.1038/s41467-019-12018-7 |
| [7] |
Li, F. J., Electrochim. Acta 2020, 332, 135457.
doi: 10.1016/j.electacta.2019.135457 |
| [8] |
Hussain, N.; Abdelkareem, M. A.; Alawadhi, H.; Elsaid, K.; Olabi, A. Chem. Eng. Sci. 2022, 258, 117757.
doi: 10.1016/j.ces.2022.117757 |
| [9] |
Kortlever, R.; Peters, I.; Koper, S.; Koper, M. T. ACS Catal. 2015, 5, 3916.
doi: 10.1021/acscatal.5b00602 |
| [10] |
(a) Zhai, J. R.; Kang, Q. L.; Liu, Q. Y.; Lai, D. W.; Lu, Q. Y.; Gao, F. J. Colloid Interface Sci. 2022, 608, 1942.
doi: 10.1016/j.jcis.2021.10.096 pmid: 31414823 |
|
(b) Zhang, T.; Verma, S.; Kim, S.; Fister, T. T.; Kenis, P. J.; Gewirth, A. A. J. Electroanal. Chem. 2020, 875, 113862.
doi: 10.1016/j.jelechem.2020.113862 pmid: 31414823 |
|
|
(c) Zhang, A.; Liang, Y. X.; Li, H. P.; Zhao, X. Y.; Chen, Y. L.; Zhang, B. Y.; Zhu, W. G.; Zeng, J. Nano Lett. 2019, 19, 6547.
doi: 10.1021/acs.nanolett.9b02782 pmid: 31414823 |
|
|
(d) Feng, J.; Gao, H.; Zheng, L.; Chen, Z.; Zhang, X. Nat. Commun. 2020, 11, 1.
doi: 10.1038/s41467-019-13993-7 pmid: 31414823 |
|
|
(e) Su, X. Z.; Jiang, Z. L.; Zhou, J.; Liu, H. J.; Zhou, D. N.; Shang, H. S.; Ni, X. M.; Peng, Z.; Yang, F.; Chen, W. X. Nat. Commun. 2022, 13, 1.
doi: 10.1038/s41467-021-27699-2 pmid: 31414823 |
|
|
(f) Lee, S.; Kim, M.; Lee, K. T.; Irvine, J. T.; Shin, T. H. Adv. Energy Mater. 2021, 11, 2100339.
doi: 10.1002/aenm.v11.24 pmid: 31414823 |
|
|
(g) Hod, I.; Sampson, M. D.; Deria, P.; Kubiak, C. P.; Farha, O. K.; Hupp, J. T. ACS Catal. 2015, 5, 6302.
doi: 10.1021/acscatal.5b01767 pmid: 31414823 |
|
|
(h) Li, Y.; Adli, N. M.; Shan, W.; Wang, M.; Zachman, M. J.; Hwang, S.; Tabassum, H.; Karakalos, S.; Feng, Z.; Wang, G. Energy Environ. Sci. 2022, 15, 2108.
doi: 10.1039/D2EE00318J pmid: 31414823 |
|
|
(i) Chu, S.; Li, X.; Robertson, A. W.; Sun, Z. Acta Phys. Chim. Sinica 2021, 37, 5.
pmid: 31414823 |
|
| [11] |
Li, X.; Qian, N. K.; Ji, L.; Wu, X. Q.; Li, J. J.; Huang, J. B.; Yan, Y. C.; Yang, D. R.; Zhang, H. Nanoscale Adv. 2022, 4, 2288.
doi: 10.1039/D2NA00141A |
| [12] |
Ren, X. X.; Gao, Y. G.; Zheng, L. R.; Wang, Z. Y.; Wang, P.; Zheng, Z. K.; Liu, Y. Y.; Cheng, H. F.; Dai, Y.; Huang, B. B. Surf. Interfaces 2021, 23, 100923.
|
| [13] |
Huang, C.; Liu, J. H.; Huang, H. H.; Xu, X. F.; Ke, Z. F. Chin. Chem. Lett. 2022, 33, 262.
doi: 10.1016/j.cclet.2021.06.046 |
| [14] |
(a) Yan, X. C.; Dong, H.; Tong, H.; Wang, Y.; Shao, L. H.; Du, Y. J.; Ge, J. T.; Fang, W. B.; Zhang, F. M. Appl. Surf. Sci. 2023, 157828.
|
|
(b) Chen, Q.; Kuang, Q.; Xie, Z. X. Acta Chim. Sinica 2021, 79, 10. (in Chinese)
doi: 10.6023/A20080384 |
|
|
(陈钱, 匡勤, 谢兆雄, 化学学报, 2021, 79, 10.)
doi: 10.6023/A20080384 |
|
| [15] |
(a) Lei, F.; Liu, W.; Sun, Y.; Xu, J.; Liu, K.; Liang, L.; Yao, T.; Pan, B.; Wei, S.; Xie, Y. Nat. Commun. 2016, 7, 12697.
doi: 10.1038/ncomms12697 |
|
(b) Li, H.; Oloman, C. J. Appl. Electrochem. 2006, 36, 1105.
doi: 10.1007/s10800-006-9194-z |
|
|
(c) Zhao, Y.; Miao, Z.; Wang, F.; Liang, M.; Liu, Y.; Wu, M.; Diao, L.; Mu, J.; Cheng, Y.; Zhou, J. J. Environ. 2021, 9, 105515.
|
|
| [16] |
Ma, L. B.; Hu, Y.; Chen, R. P.; Zhu, G. Y.; Chen, T.; Lv, H. L.; Wang, Y. R.; Liang, J.; Liu, H. X.; Yan, C. Z. Nano Energy 2016, 24, 139.
doi: 10.1016/j.nanoen.2016.04.024 |
| [17] |
(a) Wang, R. F.; Liu, Y. C.; Kong, Y. F.; Xie, P.; Zhao, S. L. Chem. Eng. J. 2023, 144049.
|
|
(b) Ning, S. L.; Wang, J. G.; Xiang, D.; Huang, S. B.; Chen, W.; Chen, S. W.; Kang, X. W. J. Catal. 2021, 399, 67.
doi: 10.1016/j.jcat.2021.04.028 |
|
| [18] |
Wang, C. Z.; Tang, X. L.; Yi, H. H.; Gao, F. Y.; Ni, S. Q.; Zhang, R. C.; Shi, Y. R. Colloids Surf., A 2021, 612, 126007.
doi: 10.1016/j.colsurfa.2020.126007 |
| [19] |
Liang, Z. J.; Fadhel, B.; Schneider, C. J.; Chaffee, A. L. Adsorption 2009, 15, 429.
doi: 10.1007/s10450-009-9192-7 |
| [20] |
Lee, J. S.; Park, G. S.; Kim, S. T.; Liu, M.; Cho, J. Angew. Chem., Int. Ed. 2013, 52, 3, 1026.
|
| [21] |
Li, W.; Chen, J. W.; Xiao, Z. L.; Xing, J. B.; Yang, C.; Qi, X. P. Carbon 2021, 174, 758.
|
| [22] |
Wu, Y. L.; Yan, H.; Dai, Y.; Zhang, B. H. J. Shanghai Univ.,Nat. Sci. Ed. 2023, 29, 2. (in Chinese)
|
|
(吴艳玲, 鄢浩, 戴扬, 张宝华, 上海大学学报(自然科学版), 2023, 29, 2).
|
|
| [23] |
Wang, C. Z.; Gao, F. Y.; Yu, Q. J.; Yi, H. H.; Ni, S. Q.; Tang, X. L. Mater. Rep. 2021, 35, 21079. (in Chinese)
|
|
(王成志, 高凤雨, 于庆君, 易红宏, 倪书权, 唐晓龙, 材料导报, 2021, 35, 21079.)
|
|
| [24] |
Long, C.; Li, X.; Guo, J.; Shi, Y. A.; Liu, S. Q.; Tang, Z. Y. Small Methods 2018, 3, 1800369.
doi: 10.1002/smtd.v3.3 |
| [25] |
Albero, J.; Peng, Y.; García, H. ACS Catal. 2020, 10, 5734.
doi: 10.1021/acscatal.0c00478 |
| [26] |
Duan, J. Y.; Liu, T. Y.; Zhao, Y. H.; Yang, R. O.; Zhao, Y.; Wang, W. B.; Liu, Y. W.; Li, H. Q.; Li, Y. F.; Zhai, T. Y. Nat. Commun. 2022, 13, 2039.
doi: 10.1038/s41467-022-29699-2 |
| [27] |
Lu, X.; Li, Z. Y.; Liu, Y. A.; Tang, B.; Zhu, Y. C.; Razal, J. M.; Pakdel, E.; Wang, J. F.; Wang, X. G. J. Cleaner Prod. 2020, 246, 118949.
doi: 10.1016/j.jclepro.2019.118949 |
| [28] |
(a) Li, Y.; Li, S.; Zhang, T.; Shi, L. L.; Liu, S. T.; Zhao, Y. J. Alloys Compd. 2019, 792, 424.
doi: 10.1016/j.jallcom.2019.03.359 |
|
(b) Zhu, H. G.; Chen, D. Y.; An, W.; Li, N. J.; Xu, Q. F.; Li, H.; He, J. H.; Lu, J. M. Small 2015, 11, 5222.
doi: 10.1002/smll.201501004 |
|
| [29] |
Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. J. Electron. Spectrosc. Relat. Phenom. 2014, 195, 145.
doi: 10.1016/j.elspec.2014.07.003 |
| [30] |
Venkataswamy, P.; Jampaiah, D.; Lin, F.; Alxneit, I.; Reddy, B. M. Appl. Surf. Sci. 2015, 349, 299.
doi: 10.1016/j.apsusc.2015.04.220 |
| [31] |
(a) Wang, G. D.; Xiang, W. J.; Liu, H. Z.; Yin, Y. Z.; Ma, D. D.; Ma, J. F. ACS Appl. Energy Mater. 2023, 6, 10155.
doi: 10.1021/acsaem.3c01880 |
|
(b) Zhang, M. X.; Peng, H.; Sun, K. J.; Xie, X.; Lei, X. F.; Liu, S. T.; Ma, G. F.; Lei, Z. Q. Chin. J. Chem. 2022, 40, 2763.
doi: 10.1002/cjoc.v40.23 |
|
| [32] |
Wang, Y. H.; Yao, C. Y.; Cao, Y. J.; Zhang, C.; Tang, W. X. Ceram. Int. 2023, 49, 1137.
doi: 10.1016/j.ceramint.2022.09.090 |
| [33] |
Zhang, P.; Wang, R. T.; He, M.; Lang, J. W.; Xu, S.; Yan, X. B. Adv. Funct. Mater. 2016, 26, 1354.
doi: 10.1002/adfm.v26.9 |
| [34] |
(a) Gan, G. Q.; Fan, S. Y.; Li, X. Y.; Wang, J.; Bai, C. P.; Guo, X. C.; Tade, M.; Liu, S. M. ACS Catal. 2021, 11, 14284.
doi: 10.1021/acscatal.1c03701 |
|
(b) Adamu, H.; Dubey, P.; Anderson, J. A. Chem. Eng. J. 2016, 284, 380.
doi: 10.1016/j.cej.2015.08.147 |
|
| [35] |
(a) Zhang, L. H.; Shi, Y. M.; Wang, Y.; Shiju, N. R. Adv. Sci. 2020, 7, 1902126.
doi: 10.1002/advs.v7.5 |
|
(b) Ortiz-Medina, J.; Wang, Z. P.; Cruz-Silva, R.; Morelos-Gomez, A.; Wang, F.; Yao, X. D.; Terrones, M.; Endo, M. Adv. Mater. 2019, 31, 1805717.
doi: 10.1002/adma.v31.13 |
|
|
(c) Tang, C.; Zhang, Q. Adv. Mater. 2017, 29, 1604103.
doi: 10.1002/adma.v29.13 |
|
| [36] |
(a) Zhu, J. W.; Huang, Y. P.; Mei, W. C.; Zhao, C. Y.; Zhang, C. T.; Zhang, J.; Amiinu, I. S.; Mu, S. C. Angew. Chem., Int. Ed. 2019, 58, 3859.
doi: 10.1002/anie.v58.12 |
|
(b) Wang, W.; Shang, L.; Chang, G. J.; Yan, C. Y.; Shi, R.; Zhao, Y. X.; Waterhouse, G. I.; Yang, D. J.; Zhang, T. R. Adv. Mater. 2019, 31, 1808276.
doi: 10.1002/adma.v31.19 |
|
| [37] |
Nethravathi, C.; Rajamathi, M. Carbon 2008, 46, 1994.
doi: 10.1016/j.carbon.2008.08.013 |
| [38] |
Xu, J.; Roghabadi, F. A.; Luo, Y.; Ahmadi, V.; Wang, Q.; Wang, Z.; He, H. J. Environ. Sci. 2023, 140, 165.
doi: 10.1016/j.jes.2023.06.028 |
| [39] |
Jia, Y. F.; Li, F.; Fan, K.; Sun, L. C. Adv. Powder Mater. 2021, 1, 1.
|
| [40] |
Birdja, Y. Y.; Pérez-Gallent, E.; Figueiredo, M. C.; Göttle, A. J.; Calle-Vallejo, F.; Koper, M. T. Nat. Energy 2019, 4, 732.
doi: 10.1038/s41560-019-0450-y |
| [41] |
Mino, L.; Spoto, G.; Ferrari, A. M. J. Phys. Chem. C. 2014, 118, 25016.
doi: 10.1021/jp507443k |
| [42] |
Tang, X. L.; Wang, C. Z.; Gao, F. Y.; Ma, Y. L.; Yi, H. H.; Zhao, S. Z.; Zhou, Y. S. J. Environ. Chem. Eng. 2020, 8, 104399.
doi: 10.1016/j.jece.2020.104399 |
| [43] |
(a) Shen, B. X.; Zhu, S. W.; Zhang, X.; Chi, G. L.; Patel, D.; Si, M.; Wu, C. F. Fuel 2018, 224, 241.
doi: 10.1016/j.fuel.2018.03.080 |
|
(b) Larrubia, M. A.; Ramis, G.; Busca, G. Appl. Catal., B 2000, 27, L145.
doi: 10.1016/S0926-3373(00)00150-8 |
|
|
(c) Zawadzki, J.; Wiśniewski, M. Carbon 2003, 41, 2257.
doi: 10.1016/S0008-6223(03)00251-3 |
|
| [44] |
Wang, F.; Xie, Z. B.; Liang, J. S.; Fang, B. Z.; Piao, Y. A.; Hao, M.; Wang, Z. S. Environ. Sci. Technol. 2019, 53, 6989.
doi: 10.1021/acs.est.9b02620 |
| [45] |
Wang, C. Z.; Gao, F. Y.; Ko, S. J.; Liu, H. H.; Yi, H. H.; Tang, X. L. Chem. Eng. J. 2022, 434, 134729.
doi: 10.1016/j.cej.2022.134729 |
| [46] |
Ho, C.; Yu, J. C.; Kwong, T.; Mak, A. C.; Lai, S. Chem. Mater. 2005, 17, 4514.
doi: 10.1021/cm0507967 |
| [47] |
Zhu, D. D.; Liu, J. L.; Qiao, S. Z. Adv. Mater. 2016, 28, 3423.
doi: 10.1002/adma.v28.18 |
| [48] |
(a) Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Chem. Rev. 2016, 116, 5987.
doi: 10.1021/acs.chemrev.5b00603 |
|
(b) Kumari, N.; Haider, M. A.; Agarwal, M.; Sinha, N.; Basu, S. J. Phys. Chem. C 2016, 120, 16626.
doi: 10.1021/acs.jpcc.6b02860 |
|
| [49] |
Wang, Y. F.; Chen, Z.; Han, P.; Du, Y. H.; Gu, Z. X.; Xu, X.; Zheng, G. F. ACS Catal. 2018, 8, 7113.
doi: 10.1021/acscatal.8b01014 |
| [50] |
Creamer, A. E.; Gao, B.; Zhang, M. Chem. Eng. J. 2014, 249, 174.
doi: 10.1016/j.cej.2014.03.105 |
| [51] |
(a) Geng, Z. G.; Kong, X. D.; Chen, W. W.; Su, H. Y.; Liu, Y.; Cai, F.; Wang, G. X.; Zeng, J. Angew. Chem., Int. Ed. 2018, 57, 6054;
doi: 10.1002/anie.v57.21 |
|
(b) Liu, L. J.; Jiang, Y. Q.; Zhao, H. L.; Chen, J. T.; Cheng, J. L.; Yang, K. S.; Li, Y. ACS Catal. 2016, 6, 1097.
doi: 10.1021/acscatal.5b02098 |
|
| [52] |
Sheng, S.; Ye, K.; Gao, Y. Y.; Zhu, K.; Yan, J.; Wang, G. L.; Cao, D. X. J. Colloid Interface Sci. 2021, 602, 325.
doi: 10.1016/j.jcis.2021.06.001 |
| [53] |
(a) Yang, X. X.; Chen, X.; Cao, H. L.; Li, C.; Wang, L. L.; Wu, Y. L.; Wang, C. Z.; Li, Y. J. Power Sources 2020, 480, 228741.
doi: 10.1016/j.jpowsour.2020.228741 |
|
(b) Li, Z. Y.; Yang, Y. S.; Ding, H.; Li, Z.; Wang, L.; Zhang, X.; Li, J.; Xie, W. F.; Hu, X. Y.; Wang, B. Chem Catal. 2023, 3, 10.
|
|
| [54] |
Zheng, W.; Nayak, S.; Yuan, W.; Zeng, Z.; Hong, X.; Vincent, K. A.; Tsang, S. C. E. Chem. Commun. 2016, 52, 13901.
doi: 10.1039/C6CC07212G |
| [55] |
Wang, J.; Zheng, M. Y.; Zhao, X.; Fan, W. L. ACS Catal. 2022, 12, 5441.
doi: 10.1021/acscatal.2c00429 |
| [56] |
(a) Wang, C.; Zhu, C.; Zhang, M.; Geng, Y.; Su, Z. Adv. Theory Simul. 2020, 3, 2000218.
doi: 10.1002/adts.v3.12 |
|
(b) Peng, L. W.; Zhang, Y.; He, R. N.; Xu, N. N.; Qiao, J. L. Acta Phys. Chim. Sinica 2023, 39, 2302037.
|
|
| [57] |
Hummers Jr, W. S.; Offeman, R. E. J. Am. Chem. Soc. 1958, 80, 1339.
doi: 10.1021/ja01539a017 |
| [1] | 张强, 王欢, 王帅, 王园园, 张梅, 宋华. NiCe(x)/FLRC-TiO2催化剂的制备及其加氢脱氧性能研究[J]. 化学学报, 2024, 82(3): 287-294. |
| [2] | 刘春梅, 高燕均, 陈鹏亮. 直接甲酸钠/铁氰化钾微流体燃料电池性能研究[J]. 化学学报, 2022, 80(9): 1256-1263. |
| [3] | 蒋银龙, 李国超, 陈青松, 徐忠宁, 林姗姗, 郭国聪. 富晶格位错的多孔铋纳米花高效电还原二氧化碳制甲酸盐※[J]. 化学学报, 2022, 80(6): 703-707. |
| [4] | 陆远, 王继芬, 谢华清. LiMn2O4尖晶石氧化物的低指数表面结构优化及表面能的第一性原理研究[J]. 化学学报, 2021, 79(8): 1058-1064. |
| [5] | 李童心, 李东林, 张清波, 高建行, 孔祥泽, 樊小勇, 苟蕾. 大孔高镍LiNi0.8Co0.1Mn0.1O2正极材料的制备及其电化学性能研究[J]. 化学学报, 2021, 79(5): 678-684. |
| [6] | 李燕丽, 于丹丹, 林森, 孙东飞, 雷自强. α-MnO2纳米棒/多孔碳正极材料的制备及水系锌离子电池性能研究[J]. 化学学报, 2021, 79(2): 200-207. |
| [7] | 梁其梅, 郭昱娇, 郭俊明, 向明武, 刘晓芳, 白玮, 宁平. 亚微米去顶角八面体LiNi0.08Mn1.92O4正极材料制备及高温电化学性能[J]. 化学学报, 2021, 79(12): 1526-1533. |
| [8] | 陈莹莹, 刘欢, 程彦, 谢青季. 动态氢气泡/牺牲铜模板法制备蜂窝AuPtCu电催化剂用于甲酸氧化[J]. 化学学报, 2020, 78(4): 330-336. |
| [9] | 代迷迷, 王健, 李麟阁, 王琪, 刘美男, 张跃钢. 界面增强的CeO2/FeNi MOF高效析氧催化剂[J]. 化学学报, 2020, 78(4): 355-362. |
| [10] | 宋波, 秦安军, 唐本忠. 绿色单体二氧化碳参与的新型聚合反应[J]. 化学学报, 2020, 78(1): 9-22. |
| [11] | 武卓敏, 石勇, 李春艳, 牛丹阳, 楚奇, 熊巍, 李新勇. 双金属MOF-74-CoMn催化剂的制备及其CO选择性催化还原技术应用[J]. 化学学报, 2019, 77(8): 758-764. |
| [12] | 孙梦佳, 吴天怡, 李天玉, 郭风巧, 唐阳, 莫恒亮, 杨志涛, 万平玉. 石墨毡载纳米δ-MnO2高性能除铵净水材料研究[J]. 化学学报, 2018, 76(6): 467-474. |
| [13] | 王一帆, 范益梅, 蹇君, 潘雨民, 赵亮, 敬雪平, 周圣家, 谌晓洪, 杜泉, 王玲, 吴小菊, 傅相锴. 酚氧基修饰的AlPS-PVPA固载手性Salen Mn(III)催化剂的合成及催化烯烃不对称环氧化研究[J]. 化学学报, 2017, 75(7): 715-722. |
| [14] | 郑卓, 吴振国, 向伟, 郭孝东. 高倍率球形锂离子电池正极材料LiNi0.5Co0.2Mn0.3O2的制备及其电化学性能研究[J]. 化学学报, 2017, 75(5): 501-507. |
| [15] | 宋聪颖, 孙逊, 叶克, 朱凯, 程魁, 闫俊, 曹殿学, 王贵领. 还原氧化石墨烯修饰泡沫镍原位负载MnO2对H2O2电还原反应催化性能的研究[J]. 化学学报, 2017, 75(10): 1003-1009. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||