Acta Chimica Sinica ›› 2025, Vol. 83 ›› Issue (6): 569-578.DOI: 10.6023/A24120367 Previous Articles Next Articles
Article
杨天宇a, 代佳楠a,b, 张玉洁a, 薛娟琴a, 马晶a,*( )
)
投稿日期:2024-12-12
发布日期:2025-04-08
基金资助:
Tianyu Yanga, Jianan Daia,b, Yujie Zhanga, Juanqin Xuea, Jing Maa,*( )
)
Received:2024-12-12
Published:2025-04-08
Contact:
*E-mail: majing@xauat.edu.cn
Supported by:Share
Tianyu Yang, Jianan Dai, Yujie Zhang, Juanqin Xue, Jing Ma. Preparation of Interlayer Anion Controllable LiAl-LDHs and Lithium Adsorption Performance[J]. Acta Chimica Sinica, 2025, 83(6): 569-578.
| Sample | Surface area/ (m2•g−1) | Pore volume/ (cm3•g−1•nm−1) | Pore diameter/nm |
|---|---|---|---|
| LiAl-LDHs | 14.03 | 0.071 | 20.141 |
| LiAl-LDHs-ST | 15.51 | 0.107 | 8.844 |
| LiAl-LDHs-SP | 105.03 | 0.340 | 28.272 |
| LiAl-LDHs-SPT | 130.28 | 0.637 | 19.218 |
| Sample | Surface area/ (m2•g−1) | Pore volume/ (cm3•g−1•nm−1) | Pore diameter/nm |
|---|---|---|---|
| LiAl-LDHs | 14.03 | 0.071 | 20.141 |
| LiAl-LDHs-ST | 15.51 | 0.107 | 8.844 |
| LiAl-LDHs-SP | 105.03 | 0.340 | 28.272 |
| LiAl-LDHs-SPT | 130.28 | 0.637 | 19.218 |
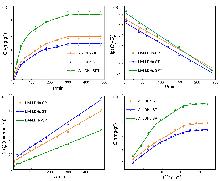

| Sample | Qe/(mg•g−1) | $Q_{e}^{*}$/(mg•g−1) | k1/min−1 | R2 |
|---|---|---|---|---|
| LiAl-LDHs-ST | 5.902 | 5.558 | 0.00418 | 0.986 |
| LiAl-LDHs-SP | 4.918 | 4.678 | 0.00427 | 0.990 |
| LiAl-LDHs-SPT | 8.852 | 7.883 | 0.00494 | 0.992 |
| Sample | Qe/(mg•g−1) | $Q_{e}^{*}$/(mg•g−1) | k1/min−1 | R2 |
|---|---|---|---|---|
| LiAl-LDHs-ST | 5.902 | 5.558 | 0.00418 | 0.986 |
| LiAl-LDHs-SP | 4.918 | 4.678 | 0.00427 | 0.990 |
| LiAl-LDHs-SPT | 8.852 | 7.883 | 0.00494 | 0.992 |
| Sample | Qe/(mg•g−1) | $Q_{e}^{*}$/(mg•g−1) | k2/(g•mg−1•min−1) | R2 |
|---|---|---|---|---|
| LiAl-LDHs-ST | 5.902 | 6.887 | 0.00188 | 0.994 |
| LiAl-LDHs-SP | 4.918 | 5.848 | 0.00206 | 0.995 |
| LiAl-LDHs-SPT | 8.852 | 9.855 | 0.00203 | 0.998 |
| Sample | Qe/(mg•g−1) | $Q_{e}^{*}$/(mg•g−1) | k2/(g•mg−1•min−1) | R2 |
|---|---|---|---|---|
| LiAl-LDHs-ST | 5.902 | 6.887 | 0.00188 | 0.994 |
| LiAl-LDHs-SP | 4.918 | 5.848 | 0.00206 | 0.995 |
| LiAl-LDHs-SPT | 8.852 | 9.855 | 0.00203 | 0.998 |
| Sample | K1a | K2a | K3a | $R_{1}^{2}$ | $R_{2}^{2}$ | $R_{3}^{2}$ |
|---|---|---|---|---|---|---|
| LiAl-LDHs-ST | 0.417 | 0.336 | 0.064 | 0.983 | 0.986 | 0.393 |
| LiAl-LDHs-SP | 0.417 | 0.250 | 0.064 | 0.983 | 0.922 | 0.393 |
| LiAl-LDHs-SPT | 0.827 | 0.379 | 0.064 | 0.986 | 0.976 | 0.393 |
| Sample | K1a | K2a | K3a | $R_{1}^{2}$ | $R_{2}^{2}$ | $R_{3}^{2}$ |
|---|---|---|---|---|---|---|
| LiAl-LDHs-ST | 0.417 | 0.336 | 0.064 | 0.983 | 0.986 | 0.393 |
| LiAl-LDHs-SP | 0.417 | 0.250 | 0.064 | 0.983 | 0.922 | 0.393 |
| LiAl-LDHs-SPT | 0.827 | 0.379 | 0.064 | 0.986 | 0.976 | 0.393 |


| Sample | Qm/(mg•g−1) | KL/min−1 | R2 |
|---|---|---|---|
| LiAl-LDHs-ST | 6.796 | 0.055 | 0.716 |
| LiAl-LDHs-SP | 5.947 | 0.045 | 0.815 |
| LiAl-LDHs-SPT | 9.281 | 0.139 | 0.850 |
| Sample | Qm/(mg•g−1) | KL/min−1 | R2 |
|---|---|---|---|
| LiAl-LDHs-ST | 6.796 | 0.055 | 0.716 |
| LiAl-LDHs-SP | 5.947 | 0.045 | 0.815 |
| LiAl-LDHs-SPT | 9.281 | 0.139 | 0.850 |
| Sample | KF/min−1 | n | R2 |
|---|---|---|---|
| LiAl-LDHs-ST | 3.352 | 13.93 | 0.914 |
| LiAl-LDHs-SP | 2.834 | 9.14 | 0.909 |
| LiAl-LDHs-SPT | 5.941 | 8.88 | 0.937 |
| Sample | KF/min−1 | n | R2 |
|---|---|---|---|
| LiAl-LDHs-ST | 3.352 | 13.93 | 0.914 |
| LiAl-LDHs-SP | 2.834 | 9.14 | 0.909 |
| LiAl-LDHs-SPT | 5.941 | 8.88 | 0.937 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
doi: 10.6023/A21070343 |
|
(赵敏, 王雪, 刘雅楠, 贺宇飞, 李殿卿, 化学学报, 2021, 79, 1518.)
doi: 10.6023/A21070343 |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
|
(王晓亮, 张多, 石雪梅, 乔心页, 程焱, 赵浩男, 常雷明, 于振秋, 黄传辉, 杨绍斌, 无机化学学报, 2023, 39, 607.)
|
|
| [29] |
|
|
(吕斌, 寇梦楠, 高党鸽, 雒康, 化工新型材料, 2023, 51, 46.)
doi: 10.19817/j.cnki.issn1006-3536.2023.04.008 |
|
| [30] |
|
|
(郑美琪, 毛方琪, 孔祥贵, 段雪, 高等学校化学学报, 2022, 43, 20220456.)
doi: 10.7503/cjcu20220456 |
|
| [31] |
|
|
(兀晓文, 杜娜, 李海平, 张人杰, 侯万国, 化学学报, 2014, 72, 963.)
doi: 10.6023/A14030146 |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
doi: 10.1016/j.jechem.2020.02.025 |
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
|
(李佳欣, 李蓓, 王纪康, 何蕾, 赵宇飞, 化学学报, 2021, 79, 238.)
doi: 10.6023/A20090441 |
|
| [41] |
|
| [42] |
|
|
(李雷, 唐鋆磊, 王丽涛, 李江涛, 全洪林, 林冰, 王宏, 李佳奇, 周太刚, 化学学报, 2024, 82, 748.)
doi: 10.6023/A24030077 |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
|
(马超, 武佳炜, 朱琳, 韩晓霞, 阮伟东, 宋薇, 王旭, 赵冰, 化学学报, 2019, 77, 1024.)
doi: 10.6023/A19050191 |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
|
(朱玉荃, 赵晓婕, 钟嫄, 陈子茹, 鄢红, 段雪, 高等学校化学学报, 2020, 41, 2287.)
doi: 10.7503/cjcu20200404 |
|
| [52] |
|
| [1] | Kaiqing Wang, Shuo Yuan, Wangdong Xu, Dan Huo, Qiulin Yang, Qingxi Hou, Dehai Yu. Preparation and Adsorption Properties of ZIF-8@B-CNF Composite Aerogel [J]. Acta Chimica Sinica, 2023, 81(6): 604-612. |
| [2] | Na Yang, Jianzhong Ma, Jiabo Shi, Xu Guo. Organic Modification of Layered Double Hydroxides and Its Applications [J]. Acta Chimica Sinica, 2023, 81(2): 207-216. |
| [3] | Yaru Wei, Jing Ma, Tingting Yuan, Jiawei Jiang, Yinli Duan, Juanqin Xue. Preparation and Adsorption Properties of Lithium Chloride Intercalation Carbon Nitride [J]. Acta Chimica Sinica, 2022, 80(4): 494-502. |
| [4] | Jiaxin Li, Bei Li, Jikang Wang, Lei He, Yufei Zhao. Recent Advances in Layered Double Hydroxides and Their Derivatives for Biomedical Applications [J]. Acta Chimica Sinica, 2021, 79(3): 238-256. |
| [5] | Si Wang, Jialing Ma, Lifang Chen, Xin Zhang. Role of Synergistic Effect in Oxygen Evolution Reaction over Layered Double Hydroxide [J]. Acta Chimica Sinica, 2021, 79(2): 216-222. |
| [6] | Wang Ning, Pang Hongwei, Yu Shujun, Gu Pengcheng, Song Shuang, Wang Hongqing, Wang Xiangke. Investigation of Adsorption Mechanism of Layered Double Hydroxides and Their Composites on Radioactive Uranium:A Review [J]. Acta Chim. Sinica, 2019, 77(2): 143-152. |
| [7] | Yu Jun, Yang Yusen, Wei Min. Preparation and Catalytic Performance of Supported Catalysts Derived from Layered Double Hydroxides [J]. Acta Chimica Sinica, 2019, 77(11): 1129-1139. |
| [8] | Jia Yunsheng, Wang Huoyan, Zhao Xuesong, Liu Xiaowei, Wang Yiliu, Fan Qunlong, Zhou Jianmin. Exploring and Evaluation of CaAl Hydrotalcite-like Adsorbents on Phosphate Recycling [J]. Acta Chim. Sinica, 2015, 73(11): 1207-1213. |
| [9] | Zhang Chao, Wang Xing, Song Xiliang, Song Kaihui, Qian Ping, Yin Hongzong. Quantum Chemical Study of Intercalation of Hydrazine Hydrate in Kaolinite [J]. Acta Chimica Sinica, 2013, 71(11): 1553-1562. |
| [10] | Zhang Xiaoqing, Zeng Meigui, Li Shuping. Influence of Different Solvents on the Property of Methotrexate/Layered Double Hydroxides [J]. Acta Chimica Sinica, 2013, 71(02): 246-254. |
| [11] | Zhu Haitao, Ni Zheming, Xue Jilong. Adsorption Applications of Acid Blue 7 on Mg/Al Layered Double Oxides and Mechanism Study [J]. Acta Chimica Sinica, 2012, 70(17): 1798-1804. |
| [12] | Liu Yuzhong, Wang Xiaoqun, Chen Yichi, Du Shanyi. Co-intercalation of Montmorillonite by Poly(oxypropylene) Amine Hydrochlorides with Different Chain Length [J]. Acta Chimica Sinica, 2012, 70(07): 911-916 . |
| [13] | Cui Wenquan, Qi Yueli, Hu Jinshan, Liu Li, Liang Yinghua. Synthesis of CdS-pillared K2La2Ti3O10 Catalyst via a Microwave Assisted Process and Its Photocatalytic Property [J]. Acta Chimica Sinica, 2012, 70(06): 691-698. |
| [14] | Gou Guojing, Wang Zhiyu, Liu Yanhong, Xue Bing, Huang Jie, Sun Yue. Supra-molecular Assembly Characterization and Urgent Toxic Level of "Dextran-Magnetic Layered Double Hydroxide-Fluorouracil" Drug Delivery System [J]. Acta Chimica Sinica, 2012, 70(02): 161-169. |
| [15] | CAO Gen-Ting, XU Qian, NI Zhe-Ming. Electronic Structure and Bond Character for Mg3Al-LDH-Cl Containing Interlayer Water [J]. Acta Chimica Sinica, 2011, 69(24): 2947-2954. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||