Acta Chimica Sinica ›› 2021, Vol. 79 ›› Issue (12): 1526-1533.DOI: 10.6023/A21070324 Previous Articles Next Articles
Article
梁其梅a, 郭昱娇b, 郭俊明a,*( ), 向明武a,*(
), 向明武a,*( ), 刘晓芳a, 白玮a, 宁平b
), 刘晓芳a, 白玮a, 宁平b
投稿日期:2021-07-13
发布日期:2021-11-02
通讯作者:
郭俊明, 向明武
基金资助:
Qimei Lianga, Yujiao Guob, Junming Guoa( ), Mingwu Xianga(
), Mingwu Xianga( ), Xiaofang Liua, Wei Baia, Ping Ningb
), Xiaofang Liua, Wei Baia, Ping Ningb
Received:2021-07-13
Published:2021-11-02
Contact:
Junming Guo, Mingwu Xiang
Supported by:Share
Qimei Liang, Yujiao Guo, Junming Guo, Mingwu Xiang, Xiaofang Liu, Wei Bai, Ping Ning. Preparation and High Temperature Electrochemical Performance of LiNi0.08Mn1.92O4 Cathode Material of Submicron Truncated Octahedron[J]. Acta Chimica Sinica, 2021, 79(12): 1526-1533.

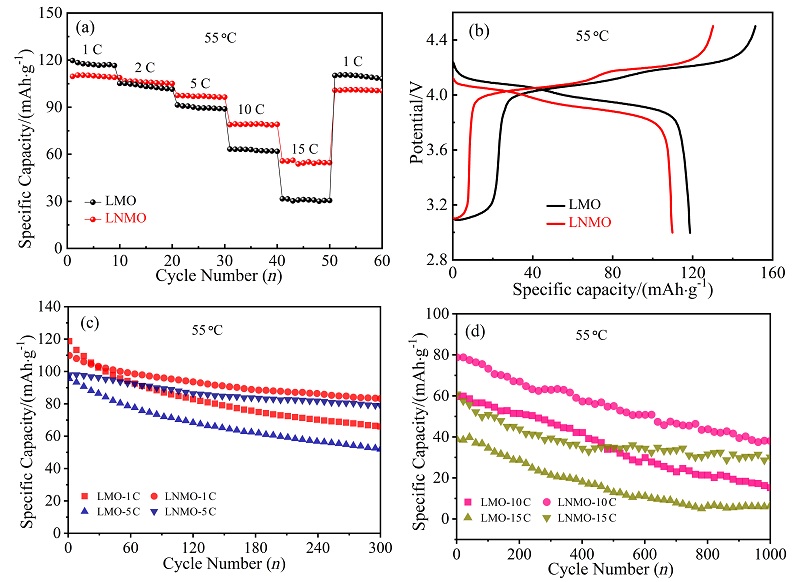

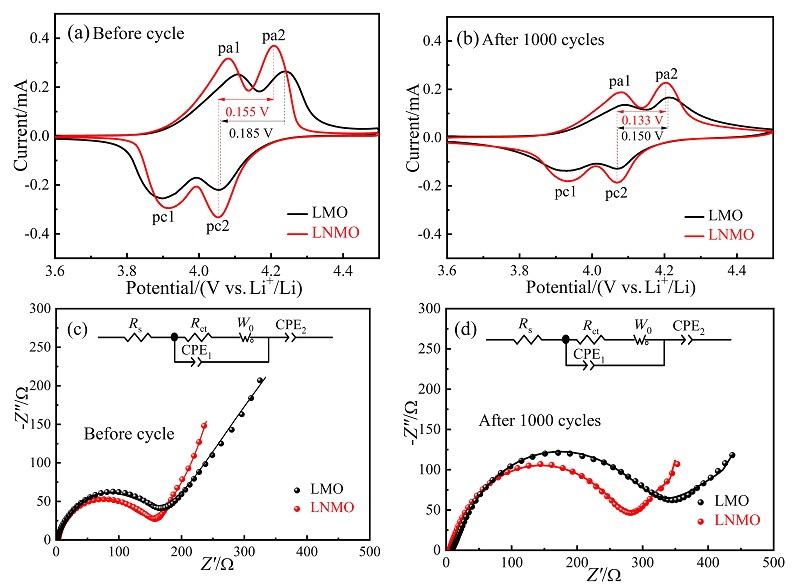


| [1] |
Chen, S.; He, T.; Su, Y.-F.; Lu, Y.; Bao, L.-L.; Chen, L.; Zhang, Q.-Y.; Wang, J.; Chen, R.-J.; Wu, F. ACS Appl. Mater. Interfaces 2017, 9, 29732.
doi: 10.1021/acsami.7b08006 |
| [2] |
Liu, J.-D.; Zhang, Y.-D.; Liu, J.-X.; Li, J.-H.; Qiu, X.-G.; Cheng, F.-Y. Acta Chim. Sinica 2020, 78, 1426 (in Chinese).
doi: 10.6023/A20070330 |
|
( 刘九鼎, 张宇栋, 刘俊祥, 李金翰, 邱晓光, 程方益, 化学学报, 2020, 78, 1426.)
doi: 10.6023/A20070330 |
|
| [3] |
Zhan, C.; Wu, T.-P.; Lu, J.; Amine, K. Energy Environ. Sci. 2018, 11, 243.
doi: 10.1039/C7EE03122J |
| [4] |
Duan, Y.-Z.; Zhu, J.-Y.; Guo, J.-M.; Xiang, M.-W.; Liu, X.-F.; Bai, H.-L.; Su, C.-W. Chem. J. Chinese Universities 2019, 40, 2574 (in Chinese).
|
|
( 段玉珍, 朱金玉, 郭俊明, 向明武, 刘晓芳, 白红丽, 苏长伟, 高等学校化学学报, 2019, 40, 2574.)
|
|
| [5] |
Lu, D.; Zheng, C.-M.; Chen, Y.-F.; Li, Y.-J.; Zhang, H.-M. Chem. J. Chinese Universities 2020, 41, 16844 (in Chinese).
|
|
( 陆地, 郑春满, 陈宇方, 李宇杰, 张红梅, 高等学校化学学报, 2020, 41, 16844.)
|
|
| [6] |
Luo, X.-Y.; Xiang, M.-W.; Li, Y.; Guo, J.-M.; Liu, X.-F.; Bai, H.-L.; Bai, W.; Su, C.-W. Vacuum 2020, 179, 109505.
doi: 10.1016/j.vacuum.2020.109505 |
| [7] |
Song, H.; Zhao, Y.; Niu, Y.; Hou, H. Solid State Ionics 2019, 331, 49.
doi: 10.1016/j.ssi.2018.12.015 |
| [8] |
Ni, J.-F.; Zhou, H.-H.; Chen, J.-T.; Su, G.-Y. Prog. Chem. 2004, 16, 335 (in Chinese).
|
|
( 倪江锋, 周恒辉, 陈继涛, 苏光耀, 化学进展, 2004, 16, 335.)
|
|
| [9] |
Iqbal, A.; Iqbal, Y.; Khan, A.-M.; Ahmed, S. Ionics 2017, 23, 1995.
doi: 10.1007/s11581-017-2062-5 |
| [10] |
Liu, H.-Q.; Tian, R.-Y.; Jiang, Y.; Tan, X.-H.; Chen, J.-K.; Zhang, L.-N.; Guo, Y.-J.; Wang, H.-F.; Sun, L.-F.; Chu, W.-G. Electrochim. Acta 2015, 180, 138.
doi: 10.1016/j.electacta.2015.08.123 |
| [11] |
Yang, S.-Q.; Zhang, T.-R.; Tao, Z.-L.; Chen, J. Acta Chim. Sinica 2013, 71, 1029 (in Chinese).
doi: 10.6023/A13030294 |
|
( 杨思七, 张天然, 陶占良, 陈军, 化学学报, 2013, 71, 1029.)
doi: 10.6023/A13030294 |
|
| [12] |
Liu, Q.; Liang, Q.-M.; Guo, J.-M.; Xiang, M.-W.; Bai, W.; Bai, H.-L.; Liu, X.-F. Ceram. Int. 2021, 47, 2441.
doi: 10.1016/j.ceramint.2020.09.086 |
| [13] |
Liu, W.-H.; Xu, H.-H.; Zhou, Q.-H.; Dai, Y.-W.; Hu, W.; Li, H.-L. J. Electron. Mater. 2020, 49, 5523.
doi: 10.1007/s11664-020-08298-1 |
| [14] |
Jiang, J.-B.; Liang, L.-W.; Li, D.; Xiao, J.; Peng, Z.-D.; Du, K.; Cao, Y.-B.; Hu, G.-R.; Jiang, F. J. Nanosci. Nanotechnol. 2017, 17, 9182.
doi: 10.1166/jnn.2017.13919 |
| [15] |
Bai, H.-L.; Xu, W.-Q.; Guo, J.-M.; Su, C.-W.; Xiang, M.-W.; Liu, X.-F.; Wang, R. J. Mater. Sci.-Mater. M. 2018, 29, 14668.
|
| [16] |
Raju, K.; Nkosi, F.-P.; Viswanathan, E.; Mathe, M.-K.; Damodaran, K.; Ozoemena, K.-I. Phys. Chem. Chem. Phys. 2016, 18, 13074.
doi: 10.1039/C6CP01873D |
| [17] |
Deepi, A.-S.; Srikesh, G.; Nesaraj, S.-A. Matéria 2021, 26, 1.
|
| [18] |
Ta, T.-A.; Nguyen, H.-S.; Nguyen, O.-T.-T.; Dang, C.-T.; Hoang, L.-A.; Pham, L.-D. Mater. Res. Express 2019, 6, 065505.
doi: 10.1088/2053-1591/ab0ab2 |
| [19] |
Wei, Q.-L.; Wang, X.-Y.; Wang, X.-Y.; Yang, X.-K.; Ju, B.-W.; Hu, B.-N.; Shu, H.-B.; Wen, W.-C.; Zhou, M.; Song, Y.-F.; Wu, H.; Hu, H. J. Mater. Chem. A 2013, 1, 4010.
doi: 10.1039/c3ta01698f |
| [20] |
Gu, X.; Li, X.-W.; Xu, L.-Q.; Xu, H.-Y.; Yang, J.; Qian, Y.-T. Int. J. Electrochem. Sci. 2012, 7, 2504.
|
| [21] |
Duan, Y.-Z.; Guo, J.-M.; Xiang, M.-W.; Zhu, J.-Y.; Su, C.-W.; Bai, H.-L.; Liu, X.-F.; Bai, W.; Wang, R. Solid State Ionics 2018, 326, 100.
doi: 10.1016/j.ssi.2018.09.014 |
| [22] |
Kunjuzwa, N.; Kebede, M.; Ozoemena, K.-I.; Mathe, M.-K. RSC Adv. 2016, 6, 111882.
doi: 10.1039/C6RA23052K |
| [23] |
Wang, F.-X.; Xiao, S.-Y.; Shi, Y.; Liu, L.-L.; Zhu, Y.-S.; Wu, Y.-P.; Wang, J.-Z.; Holze, R. Electrochim. Acta 2013, 93, 301.
doi: 10.1016/j.electacta.2013.01.106 |
| [24] |
Zhao, C.-H.; Kang, W.-P.; Wang, X.-X.; Zhao, S.-Q.; Shen, Q. Micro Nano Lett. 2012, 7, 558.
doi: 10.1049/mnl.2012.0020 |
| [25] |
Benedek, R.; Thackeray, M.-M. J. Phys. Chem. C 2012, 116, 4050.
doi: 10.1021/jp208793k |
| [26] |
Kim, J.-S.; Kim, K. S.; Cho, W.; Shin, W.-H.; Kanno, R. Nano Lett. 2012, 12, 6358.
doi: 10.1021/nl303619s |
| [27] |
Huang, S.-S.; Wu, H.; Chen, P.-H.; Guo, Y.; Nie, B.; Chen, B.-J.; Liu, H.; Zhang, Y. J. Mater. Chem. A 2015, 3, 3633.
doi: 10.1039/C4TA06522K |
| [28] |
Zhou, S.-Y.; Mei, T.; Wang, X.-B.; Qian, Y.-T. Nanoscale 2018, 10, 17435.
doi: 10.1039/C8NR04842H |
| [29] |
Jiang, C.-H.; Tang, Z.-L.; Wang, S.-T.; Zhang, Z.-T. J. Power Sources 2017, 357, 144.
doi: 10.1016/j.jpowsour.2017.04.079 |
| [30] |
Wang, F.-X.; Xiao, S.-Y.; Shi, Y.; Liu, L.-L.; Zhu, Y.-S.; Wu, Y.-P.; Wang, J.-Z.; Holze, R. Electrochim. Acta 2013, 93, 301.
doi: 10.1016/j.electacta.2013.01.106 |
| [31] |
Wang, Q.-Q.; Zhang, Y.; Zhang, H.; Xu, Y.-L.; Dong, H.; Zhao, C.-J. J. Alloys Compd. 2017, 693, 474.
doi: 10.1016/j.jallcom.2016.09.130 |
| [32] |
Zhu, C.-Y.; Liu, J.-X.; Yu, X.-H.; Zhang, Y.-J.; Dong, P.; Wang, X.; Zhang, Y.-N. Ceram Int. 2019, 45, 19351.
doi: 10.1016/j.ceramint.2019.06.187 |
| [33] |
Cai, Y.-J.; Huang, Y.-D.; Wang, X.-C.; Jia, D.-Z.; Pang, W.-K.; Guo, Z.-P.; Du, Y.-P.; Tang, X.-C. J. Power Sources 2015, 278, 574.
doi: 10.1016/j.jpowsour.2014.12.082 |
| [34] |
Tabassam, L.; Nazir, T.; Manzoor, U.; Mehmood, S.; Hassan, M.-U.; Bhatti, A.-S. Mater. Res. Express 2019, 6, 115550.
doi: 10.1088/2053-1591/ab3970 |
| [35] |
Yu, Y.; Xiang, M.-W.; Guo, J.-M.; Su, C.-W.; Liu, X.-F.; Bai, H.-L.; Bai, W.; Duan, K.-J. J. Colloid Interf. Sci. 2019, 555, 64.
doi: 10.1016/j.jcis.2019.07.078 |
| [36] |
Huang, X.-H.; Li, G.-H.; Cao, B.-Q.; Wang, M.; Hao, C.-Y. J. Phys. Chem. C 2014, 113, 4381.
doi: 10.1021/jp810790h |
| [37] |
Chen, M.-F.; Chen, P.; Yang, F.; Song, H.-Y.; Liao, S.-J. Electrochim. Acta 2016, 206, 356.
doi: 10.1016/j.electacta.2016.04.148 |
| [38] |
Huang, Y.-D.; Jiang, R.-R.; Jia, D.-Z.; Guo, Z.-P. Mater. Lett. 2011, 65, 3486.
doi: 10.1016/j.matlet.2011.07.091 |
| [39] |
Eriksson, T.; Hjelm, A.-K.; Lindbergh, G.; Gustafsson, T. J. Electrochem. Soc. 2002, 149, A1164.
doi: 10.1149/1.1497170 |
| [40] |
Xia, Y.; Wang, H.; Zhang, Q.; Nakamura, H.; Noguchi, H.; Yoshio, M. J. Power Sources 2007, 166, 485.
doi: 10.1016/j.jpowsour.2007.01.023 |
| [41] |
Yoshio, M.; Noguchi, H.; Wang, H.; Wang, X. J. Power Sources 2006, 154, 273.
doi: 10.1016/j.jpowsour.2005.03.232 |
| [42] |
Wu, Y.; Cao, C.-B.; Zhang, J.-T.; Wang, L.; Ma, X.-L.; Xu, X.-Y. ACS Appl. Mater. Interfaces 2016, 8, 19567.
doi: 10.1021/acsami.6b06820 |
| [43] |
Li, Y.-L.; Yu, D.-D.; Lin, S.; Sun, D.-F.; Lei, Z.-Q. Acta Chim. Sinica 2021, 79, 200 (in Chinese).
doi: 10.6023/A20090428 |
|
( 李燕丽, 于丹丹, 林森, 孙东飞, 雷自强, 化学学报, 2021, 79, 200.)
doi: 10.6023/A20090428 |
|
| [44] |
Rangarajan, S.-P.; Barsukov, Y.; Mukherjee, P.-P. J. Electrochem. Soc. 2019, 166, A2131.
doi: 10.1149/2.1191910jes |
| [1] | Jiawei He, Liu Jiao, Xueyi Cheng, Guanghai Chen, Qiang Wu, Xizhang Wang, Lijun Yang, Zheng Hu. Structural Regulation of Metal Organic Framework-derived Hollow Carbon Nanocages and Their Lithium-Sulfur Battery Performance [J]. Acta Chimica Sinica, 2022, 80(7): 896-902. |
| [2] | Shouxiao Chen, Junke Liu, Weichen Zheng, Guozhen Wei, Yao Zhou, Juntao Li. Electron/ion Conductor Double-coated LiNi0.8Co0.1Mn0.1O2 Li-ion Battery Cathode Material and Its Electrochemical Performance [J]. Acta Chimica Sinica, 2022, 80(4): 485-493. |
| [3] | Xiaolan Xue, Yang Zhang, Meiyu Shi, Tianlin Li, Tianlong Huang, Jiqiu Qi, Fuxiang Wei, Yanwei Sui, Zhong Jin. Recent Progress on Organic Electrode Materials for Nonaqueous Magnesium Secondary Batteries [J]. Acta Chimica Sinica, 2022, 80(12): 1618-1628. |
| [4] | Kai Qiu, Mingxia Yan, Shouwang Zhao, Shengli An, Wei Wang, Guixiao Jia. Theoretical Study on the Structural Stability and Oxygen Ion Oxidation of Al-doped Lithium-ion Battery Layered Cathode Li(Li0.17Ni0.17Al0.04Fe0.13Mn0.49)O2 [J]. Acta Chimica Sinica, 2021, 79(9): 1146-1153. |
| [5] | Yuan Lu, Jifen Wang, Huaqing Xie. First-principles Study on Low Index Surface Structure Optimization and Surface Energy of LiMn2O4 Spinel Oxides [J]. Acta Chimica Sinica, 2021, 79(8): 1058-1064. |
| [6] | Tongxin Li, Donglin Li, Qingbo Zhang, Jianhang Gao, Xiangze Kong, Xiaoyong Fan, Lei Gou. Preparation and Electrochemical Performance of Macroporous Ni-rich LiNi0.8Co0.1Mn0.1O2 Cathode Material [J]. Acta Chimica Sinica, 2021, 79(5): 678-684. |
| [7] | Bixia Lin, Yingshan Huang, Shuai Chen, Zhenyu Xing. Research Progress of Key Materials for Sodium-selenium Batteries [J]. Acta Chimica Sinica, 2021, 79(5): 641-648. |
| [8] | Lu Zhang, Wenfeng Wang, Hongming Zhang, Shumin Han, Limin Wang. Research Progress and Challenge of Aqueous Zinc Ion Battery [J]. Acta Chimica Sinica, 2021, 79(2): 158-175. |
| [9] | Yanli Li, Dandan Yu, Sen Lin, Dongfei Sun, Ziqiang Lei. Preparation of α-MnO2 Nanorods/Porous Carbon Cathode for Aqueous Zinc-ion Batteries [J]. Acta Chimica Sinica, 2021, 79(2): 200-207. |
| [10] | Jisheng Xie, Zhumei Xiao, Wenhua Zuo, Yong Yang. Research Progresses of Sodium Cobalt Oxide as Cathode in Sodium Ion Batteries [J]. Acta Chimica Sinica, 2021, 79(10): 1232-1243. |
| [11] | Tang Gong-ao, Mao Kun, Zhang Jing, Lyu Pin, Cheng Xueyi, Wu Qiang, Yang Lijun, Wang Xizhang, Hu Zheng. Hierarchical Nitrogen-doped Carbon Nanocages as High-rate Long-life Cathode Material for Rechargeable Magnesium Batteries [J]. Acta Chimica Sinica, 2020, 78(5): 444-450. |
| [12] | Liu Jiuding, Zhang Yudong, Liu Junxiang, Li Jinhan, Qiu Xiaoguang, Cheng Fangyi. In-situ Li3PO4 Coating of Li-Rich Mn-Based Cathode Materials for Lithium-ion Batteries [J]. Acta Chimica Sinica, 2020, 78(12): 1426-1433. |
| [13] | Ren Xuqiang, Li Donglin, Zhao Zhenzhen, Chen Guangqi, Zhao Kun, Kong Xiangze, Li Tongxin. Dual Effect of Aluminum Doping and Lithium Tungstate Coating on the Surface Improves the Cycling Stability of Lithium-rich Manganese-based Cathode Materials [J]. Acta Chimica Sinica, 2020, 78(11): 1268-1274. |
| [14] | Song Xuexi, Li Jicheng, Li Zhaohui, Li Xifei, Ding Yanhuai, Xiao Qizhen, Lei Gangtie. Effect of K-Doping on the Sodium-storage Performance of Sodium Vanadate Nanoplates [J]. Acta Chim. Sinica, 2019, 77(7): 625-633. |
| [15] | Li Zhao, Wang Zhong, Ban Liqin, Wang Jiantao, Lu Shigang. Recent Advances on Surface Modification of Li- and Mn-Rich Cathode Materials [J]. Acta Chimica Sinica, 2019, 77(11): 1115-1128. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||