Acta Chimica Sinica ›› 2022, Vol. 80 ›› Issue (6): 756-764.DOI: 10.6023/A21120552 Previous Articles Next Articles
Article
毕文超, 张琳锋, 陈健, 田瑞雪, 黄昊*( ), 姚曼*(
), 姚曼*( )
)
投稿日期:2021-12-09
发布日期:2022-07-07
通讯作者:
黄昊, 姚曼
基金资助:
Wenchao Bi, Linfeng Zhang, Jian Chen, Ruixue Tian, Hao Huang( ), Man Yao(
), Man Yao( )
)
Received:2021-12-09
Published:2022-07-07
Contact:
Hao Huang, Man Yao
Supported by:Share
Wenchao Bi, Linfeng Zhang, Jian Chen, Ruixue Tian, Hao Huang, Man Yao. Lithiation Mechanism and Performance of Monoclinic ZnP2 Anode Materials[J]. Acta Chimica Sinica, 2022, 80(6): 756-764.
| Metal phosphide | P/M | Classification | Capacity/(mAh•g–1) |
|---|---|---|---|
| FeP2 | >1 (高磷比) | 单活性 | 1365[ |
| CuP2 | >1 (高磷比) | 单活性 | 1280[ |
| GeP5 | >1 (高磷比) | 双活性 | 2266[ |
| ZnP2 | >1 (高磷比) | 双活性 | 1477 |
| Ni2P | ≤1 (低磷比) | 单活性 | 542[ |
| Sn4P3 | ≤1 (低磷比) | 双活性 | 1255[ |
| Metal phosphide | P/M | Classification | Capacity/(mAh•g–1) |
|---|---|---|---|
| FeP2 | >1 (高磷比) | 单活性 | 1365[ |
| CuP2 | >1 (高磷比) | 单活性 | 1280[ |
| GeP5 | >1 (高磷比) | 双活性 | 2266[ |
| ZnP2 | >1 (高磷比) | 双活性 | 1477 |
| Ni2P | ≤1 (低磷比) | 单活性 | 542[ |
| Sn4P3 | ≤1 (低磷比) | 双活性 | 1255[ |
| Bond length/nm | ||||||
|---|---|---|---|---|---|---|
| Zn(1)—P(1) | Zn(1)—P(2) | Zn(2)—P(3) | Zn(2)—P(4) | P(1)—P(3) | P(2)—P(4) | |
| ZnP2 | 0.237 | 0.244 | 0.244 | 0.237 | 0.223 | 0.223 |
| LiZnP2 | 0.236 | 0.244 | 0.235 | 0.262 | 0.221 | 0.230 |
| Li3ZnP2 | 0.238 | 0.240 | 0.234 | 0.400 | 0.399 | 0.389 |
| Li5ZnP2 | 0.536 | 0.226 | 0.231 | 0.406 | 0.675 | 0.389 |
| Li7ZnP2 | 0.5639 | 0.461 | 0.250 | 0.411 | 0.529 | 0.403 |
| Bond length/nm | ||||||
|---|---|---|---|---|---|---|
| Zn(1)—P(1) | Zn(1)—P(2) | Zn(2)—P(3) | Zn(2)—P(4) | P(1)—P(3) | P(2)—P(4) | |
| ZnP2 | 0.237 | 0.244 | 0.244 | 0.237 | 0.223 | 0.223 |
| LiZnP2 | 0.236 | 0.244 | 0.235 | 0.262 | 0.221 | 0.230 |
| Li3ZnP2 | 0.238 | 0.240 | 0.234 | 0.400 | 0.399 | 0.389 |
| Li5ZnP2 | 0.536 | 0.226 | 0.231 | 0.406 | 0.675 | 0.389 |
| Li7ZnP2 | 0.5639 | 0.461 | 0.250 | 0.411 | 0.529 | 0.403 |


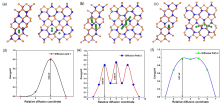



| [1] |
Choi, J. W.; Aurbach, D. Nat. Rev. Mater. 2016, 1, 16013.
doi: 10.1038/natrevmats.2016.13 |
| [2] |
Goodenough, J. B.; Park, K. S. J. Am. Chem. Soc. 2013, 135, 1167.
doi: 10.1021/ja3091438 pmid: 23294028 |
| [3] |
Lu, L.; Han, X.; Li, J.; Ouyang, M. J. Power Sources 2013, 226, 272.
doi: 10.1016/j.jpowsour.2012.10.060 |
| [4] |
Qiu, K.; Yan, M. X.; Zhao, S. W.; An, S. L.; Wang, W.; Jia, G. X. Acta Chim. Sinica 2021, 79, 1146. (in Chinese)
doi: 10.6023/A21040178 |
|
(邱凯, 严铭霞, 赵守旺, 安胜利, 王玮, 贾桂霄, 化学学报, 2021, 79, 1146.)
doi: 10.6023/A21040178 |
|
| [5] |
Tarascon, J. M.; Armand, M. Nature 2001, 414, 359.
doi: 10.1038/35104644 |
| [6] |
Zhou, X.; Liu, Q.; Jiang, C.; Ji, B.; Ji, X.; Tang, Y.; Cheng, H.-M. Angew. Chem., nt. Ed. 2019, 59, 3802.
|
| [7] |
Li, T. X.; Li, D. L.; Zhang, Q. B.; Gao, J. H.; Kong, X. Z.; Fan, X. Y.; Gou, L. Acta Chim. Sinica 2021, 79, 678. (in Chinese)
doi: 10.6023/A21010019 |
|
(李童心, 李东林, 张清波, 高建行, 孔祥泽, 樊小勇, 苟蕾, 化学学报, 2021, 79, 678.)
doi: 10.6023/A21010019 |
|
| [8] |
Dahn, J. R.; Zheng, T.; Liu, Y.; Xue, J. Science 1995, 270, 590.
doi: 10.1126/science.270.5236.590 |
| [9] |
Zheng, S. Y.; Dong, F.; Pang, Y. P.; Han, P.; Yang, J. J. Inorg. Mater. 2020, 35, 1295. (in Chinese)
doi: 10.15541/jim20200134 |
|
(郑时有, 董飞, 庞越鹏, 韩盼, 杨俊和, 无机材料学报, 2020, 35, 1295.)
doi: 10.15541/jim20200134 |
|
| [10] |
Marino, C.; Debenedetti, A.; Fraisse, B.; Favier, F.; Monconduit, L. Electrochem. Commun. 2011, 13, 346.
doi: 10.1016/j.elecom.2011.01.021 |
| [11] |
Wang, S. L.; Yang, G. R.; SalmanNasir, M.; Wang, X. J.; Wang, J. N.; Yan, W. Acta Phys.-Chim. Sin. 2021, 37, 28. (in Chinese)
|
|
(王思岚, 杨国锐, SalmanNasir, Muhammad, 王筱珺, 王嘉楠, 延卫, 物理化学学报, 2021, 37, 28.)
|
|
| [12] |
Sun, J.; Zheng, G.; Lee, H.; Liu, N.; Wang, H.; Yao, H.; Yang, W.; Cui, Y. Nano Lett. 2014, 14, 4573.
doi: 10.1021/nl501617j |
| [13] |
Kim, Y.; Park, Y.; Choi, A.; Choi, N. S.; Kim, J.; Lee, J.; Ji, H. R. Adv. Mater. 2013, 25, 3010.
doi: 10.1002/adma.201370143 |
| [14] |
Puziy, O.; Poddubnaya, A.; Martnez-Alonso, F.; Suarez-Garca; Tascon, J. M. D. Carbon 2002, 40, 1507.
doi: 10.1016/S0008-6223(01)00318-9 |
| [15] |
Jing, B.; Xi, B.; Mao, H.; Lin, Y.; Ma, X.; Feng, J.; Xiong, S. Adv. Mater. 2018, 1802310.
|
| [16] |
Wu, C.; Kopold, P.; Aken, P. A. V.; Maier, J.; Yu, Y. Adv. Mater. 2017, 29, 1604015.
doi: 10.1002/adma.201604015 |
| [17] |
Hou, B. H.; Wang, Y. Y.; Ning, Q. L.; Fan, C. Y.; Xi, X. T.; Yang, X. Nanoscale 2019, 11, 1304.
doi: 10.1039/C8NR08849G |
| [18] |
Wang, X.; Chen, K.; Wang, G.; Liu, X.; Wang, H. ACS Nano 2017, 11, 11602.
doi: 10.1021/acsnano.7b06625 |
| [19] |
Pralong, V.; Souza, D.; Leung, K. T.; Nazar, L. F. Electrochem. Commun. 2002, 4, 516.
doi: 10.1016/S1388-2481(02)00363-6 |
| [20] |
Hall, J. W.; Membreno, N.; Jing, W.; Celio, H.; Jones, R. A. J. Am. Chem. Soc. 2012, 134, 5532.
doi: 10.1021/ja301173q |
| [21] |
Kim, K. H.; Hong, S. H. Adv. Energy Mater. 2021, 11, 2003609.
doi: 10.1002/aenm.202003609 |
| [22] |
Hayashi, A.; Inoue, A.; Tatsumisago, M. J. Power Sources 2009, 189, 669.
doi: 10.1016/j.jpowsour.2008.09.047 |
| [23] |
Kim, S. O.; Manthira, A. ACS Appl. Mater. Interfaces 2017, 9, 16221.
doi: 10.1021/acsami.7b02826 |
| [24] |
Chen, M.; Zhou, W.; Qi, M.; Yin, J.; Xia, X. J. Power Sources 2017, 342, 964.
doi: 10.1016/j.jpowsour.2017.01.014 |
| [25] |
Pfeiffer, H.; Tancret, F.; Brousse, T. Electrochim. Acta 2005, 50, 4763.
doi: 10.1016/j.electacta.2005.02.024 |
| [26] |
Lu, Y.; Wang, X.; Mai, Y.; Xiang, J.; Zhang, H.; Li, L.; Gu, C.; Tu, J.; Mao, S. X. J. Phys. Chem. C 2012, 116, 22217.
doi: 10.1021/jp3073987 |
| [27] |
Liu, J.; Sun, W.; Ran, Y.; Zhou, S.; Zhang, L.; Wu, A.; Huang, H.; Yao, M. Appl. Surf. Sci. 2021, 550, 149247.
doi: 10.1016/j.apsusc.2021.149247 |
| [28] |
Li, W.; Li, H.; Lu, Z.; Gan, L.; Ke, L.; Zhai, T.; Zhou, H. Energy Environ. Sci. 2015, 8, 3629.
doi: 10.1039/C5EE02524A |
| [29] |
Hwang, H.; Kim, M. G.; Kim, Y.; Martin, S. W.; Cho, J. Energy Environ. Sci. J. Mater. Chem. 2007, 3161.
|
| [30] |
Park, C.; Sohn, H. Chem. Mater. 2008, 20, 6319.
doi: 10.1021/cm800632f |
| [31] |
Liu, J.; Wu, A.; Tian, R.; Camacho, R. P.; Zhou, S.; Huang, S.; Yao, M. Mater. Today Energy 2020, 18, 100545.
|
| [32] |
Jain, A.; Ong, S. P.; Hautier, G.; Chen, W.; Richards, W. D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; Persson, K. A. APL Mater. 2013, 1, 011002.
doi: 10.1063/1.4812323 |
| [33] |
Fleet, M. E.; White, J. C. J. Mater. Res. 1986, 1, 187.
doi: 10.1557/JMR.1986.0187 |
| [34] |
Tian, R.; Liu, C.; Zhang, G.; Wu, A.; Yao, M.; Huang, H. Appl. Surf. Sci. 2021, 553, 149448.
doi: 10.1016/j.apsusc.2021.149448 |
| [35] |
Manju, M. S.; Thomas, S.; Lee, S. U.; Madam, A. K. Appl. Surf. Sci. 2020, 541, 148417.
doi: 10.1016/j.apsusc.2020.148417 |
| [36] |
Butler, K.; Gautam, G. S.; Canepa, P. NPJ Comput Mater. 2019, 5, 19.
doi: 10.1038/s41524-019-0160-9 |
| [37] |
Zhang, Z. F.; Yu, Q. Y.; Wu, L.; Sun, L. J.; Peng, J. H. J. Chongqing Univ. 2012, 35, 83. (in Chinese)
|
|
(张正富, 余秋雁, 伍林, 孙力军, 彭金辉, 重庆大学学报, 2012, 35, 83.)
|
|
| [38] |
Fleet, M. E.; Mowles, T. A. Acta Crystallogr. 1984, 40, 1778.
|
| [39] |
Aierken, Y.; Sevik, C.; Gulseren, O.; Peeters, F. M.; Cakir, D. J. Mater. Chem. A 2018, 6, 2337.
doi: 10.1039/C7TA09001C |
| [40] |
Li, P. J.; Zhou, W. W.; Tang, Y. H.; Zhang, H.; Shi, S. Q. Acta Phys. Sin. 2010, 6. (in Chinese)
|
|
(李沛娟, 周薇薇, 唐元昊, 张华, 施思齐, 物理学报, 2010, 6.)
|
|
| [41] |
Fan, C. L.; Cheng, X. L.; Zhang, H. Phys. Status Solidi 2010, 246, 77.
doi: 10.1002/pssb.200844007 |
| [42] |
Henkelman, G.; Uberuaga, B. P. J. Phys. Chem. C 2000, 113, 9901.
|
| [43] |
Harper, A. F.; Evans, M. L.; Darby, J. P.; Bora, K.; Koer, C. P.; Nelson, J. R.; Morris, A. J. Johnson Matthey Technol. Rev. 2020, 64, 103.
doi: 10.1595/205651320X15742491027978 |
| [44] |
Lin, C. J.; Zheng, F.; Zhu, Z. Z. Acta Phys. Sin. 2019, 68, 8. (in Chinese)
|
|
(林传金, 郑锋, 朱梓忠, 物理学报, 2019, 68, 8.)
|
|
| [45] |
Chen, H.; Hua, Y.; Luo, N.; He, X.; Li, Y.; Zhang, Y.; Chen, W.; Huang, S. J. Phys. Chem. C 2020, 124, 7031.
doi: 10.1021/acs.jpcc.0c00103 |
| [46] |
Zhao, S.; Kang, W.; Xue, J. J. Mater. Chem. A 2014, 2, 19046.
doi: 10.1039/C4TA04368E |
| [47] |
Hardikar, R. P.; Das, D.; Han, S. S.; Lee, K. R.; Singh, A. K. Phys. Chem. Chem. Phys. 2014, 16, 16502.
doi: 10.1039/c4cp01412j pmid: 24986702 |
| [48] |
Zhang, W.; Liu, S.; Chen, J.; Hu, F.; Wang, X.; Huang, H.; Yao, M. ACS Appl. Mater. Interfaces 2021, 13, 22341.
doi: 10.1021/acsami.1c02470 |
| [49] |
Fang, Y.; Zhang, Y.; Miao, C.; Zhu, K.; Chen, Y.; Du, F.; Yin, J.; Ye, K.; Cheng, K.; Yan, J.; Wang, G.; Cao, D. Nano-micro. Lett. 2020, 12, 128.
|
| [50] |
Du, F.; Jin, X.; Chen, J.; Hua, Y.; Cao, M.; Zhang, L.; Li, J.; Zhang, L.; Jin, J.; Wu, C. J. Nanopart. Res. 2014, 16, 1.
|
| [51] |
Kim, Y. U.; Lee, C. K.; Kang, T. J. Electrochem. Soc. 2004, 151, A933.
doi: 10.1149/1.1738679 |
| [52] |
Berland, K.; Hyldgaard, P. Phys. Rev. B 2014, 89, 035412.
doi: 10.1103/PhysRevB.89.035412 |
| [53] |
Kresse, G. G.; Furthmüller, J. J. Phys. Rev. B 1996, 54, 11169.
doi: 10.1103/physrevb.54.11169 pmid: 9984901 |
| [54] |
Chl, P. Phys. Rev. B 1994, 50, 1795.
|
| [55] |
Paier, J.; Hirschl, R.; Marsman, M.; Georg, K. J. Phys. Chem. C 2005, 122, 234102.
|
| [56] |
Broderick, S. R.; Rajan, K. Europhys. Lett. 2011, 95, 57005.
doi: 10.1209/0295-5075/95/57005 |
| [57] |
Momma, K.; Izumi, F. J. Appl. Crystallogr. 2011, 44, 1272.
doi: 10.1107/S0021889811038970 |
| [1] | Guanglong Huang, Xiao-Song Xue. Computational Study on the Mechanism of Chen’s Reagent as Trifluoromethyl Source [J]. Acta Chimica Sinica, 2024, 82(2): 132-137. |
| [2] | Jia Yanggang, Chen Shijie, Shao Xia, Cheng Jie, Lin Na, Fang Daolai, Mao Aiqin, Li Canhua. Preparation and High-performance Lithium-ion Storage of Cobalt-free Perovskite High-entropy Oxide Anode Materials [J]. Acta Chimica Sinica, 2023, 81(5): 486-495. |
| [3] | Wang Xiaoyu, Zhang Yu, Ma Lei, Wei Liangming. Recent Development on Binders for Silicon-Based Anodes in Lithium-Ion Batteries [J]. Acta Chim. Sinica, 2019, 77(1): 24-40. |
| [4] | Wang Ling, Yang Guorui, Wang Jianan, Wang Silan, Peng Shengjie, Yan Wei. Research Progress on Electrospun Materials for Sodium-Ion Batteries [J]. Acta Chim. Sinica, 2018, 76(9): 666-680. |
| [5] | Mu Weihua, Ma Yao, Fang Decai, Wang Rong, Zhang Haina. Computational Insights into the Diels-Alder-alike Reactions of 1-Iodo-2-Lithio-o-Carborane with Fulvenes [J]. Acta Chim. Sinica, 2018, 76(1): 55-61. |
| [6] | Xiang Xingde, Lu Yanying, Chen Jun. Advance and Prospect of Functional Materials for Sodium Ion Batteries [J]. Acta Chim. Sinica, 2017, 75(2): 154-162. |
| [7] | Du Jin, Lin Ning, Qian Yitai. Recent Development of the Synthetic Method for Si/Graphite Anode Materials [J]. Acta Chimica Sinica, 2017, 75(2): 147-153. |
| [8] | Wang Lei, Zhao Dongdong, Liu Xu, Yu Peng, Fu Honggang. Hydrothermal for Synthesis of CoO Nanoparticles/Graphene Composite as Li-ion Battery Anodes [J]. Acta Chim. Sinica, 2017, 75(2): 231-236. |
| [9] | Chen Xin, Yan Huijun, Xia Dingguo. Germanium Nanotube as the Catalyst for Oxygen Reduction Reaction: Performance and Mechanism [J]. Acta Chim. Sinica, 2017, 75(2): 189-192. |
| [10] | Yang Yinuo, Zhang Qi, Shi Jing, Fu Yao. Mechanism Study of Mn(I) Complex-catalyzed Imines and Alkynes Dehydrogenation Coupling Reaction [J]. Acta Chim. Sinica, 2016, 74(5): 422-428. |
| [11] | Tong Zhenkun, Fang Shan, Zheng Hao, Zhang Xiaogang. Zn2GeO4 Nanorods@Graphene Composite as Anode Materials for Li-ion Batteries [J]. Acta Chim. Sinica, 2016, 74(2): 185-190. |
| [12] | Luo Fei, Zheng Jieyun, Chu Geng, Liu Bonan, Zhang Sulin, Li Hong, Chen Liquan. Self-healing Behavior of High Capacity Metal Gallium Thin Film and Powder as Anode Material for Li-ion Battery [J]. Acta Chim. Sinica, 2015, 73(8): 808-814. |
| [13] | Ye Ya, Zhu Jingyi, Yao Yinan, Wang Yuguo, Wu Ping, Tang Yawen, Zhou Yiming, Lu Tianhong. One-pot Synthesis of Sn/Mesoporous Carbon Composite in a Polyol System with Well-improved Lithium Storage Capability [J]. Acta Chim. Sinica, 2015, 73(2): 151-155. |
| [14] | Lyu Zhiyang, Feng Rui, Zhao Jin, Fan Hao, Xu Dan, Wu Qiang, Yang Lijun, Chen Qiang, Wang Xizhang, Hu Zheng. Nitrogen-Doped Carbon Nanocages as High-Rate Anode for Lithium Ion Batteries [J]. Acta Chim. Sinica, 2015, 73(10): 1013-1017. |
| [15] | Wen Lei, Liu Chengming, Song Rensheng, Luo Hongze, Shi Ying, Li Feng, Cheng Huiming. Lithium Storage Characteristics and Possible Applications of Graphene Materials [J]. Acta Chimica Sinica, 2014, 72(3): 333-344. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||