Acta Chimica Sinica ›› 2024, Vol. 82 ›› Issue (2): 146-151.DOI: 10.6023/A23110498 Previous Articles Next Articles
Article
投稿日期:2023-11-14
发布日期:2024-01-17
作者简介:基金资助:
Xiaoyu Gua, Jin Lia, Qian Sunb, Chaoyang Wangb( )
)
Received:2023-11-14
Published:2024-01-17
Contact:
E-mail: About author:Supported by:Share
Xiaoyu Gu, Jin Li, Qian Sun, Chaoyang Wang. Microcalorimetry Analysis of Thermal Runaway Process in Lithium-ion Batteries[J]. Acta Chimica Sinica, 2024, 82(2): 146-151.


| LFP/NCM | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于电极 产热/(J•g-1) |
|---|---|---|---|---|
| peak 1 | 130 | 180 | 84 | 1408 |
| peak 2 | 174 | 205 | 299 | |
| peak 3 | 213 | 228 | 140 | 2293 |
| peak 4 | 231 | 236 | 267 | |
| peak 5 | 241 | 245 | 29 | |
| peak 6 | 280 | 287 | 21 | 152 |
| NCM | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于电极 产热/(J•g-1) |
| peak 1 | 154 | 180 | 66 | 1632 |
| peak 2 | 185 | 208 | 378 | |
| peak 3 | 211 | 224 | 294 | 1285 |
| peak 4 | 232 | 235 | 109 | |
| peak 5 | 226 | 236 | 249 | |
| peak 6 | — | — | — | |
| LFP | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于电极 产热/(J•g-1) |
| peak 1 | 160 | 168 | 8 | 448 |
| peak 2 | 180 | 209 | 65 | |
| peak 3 | 197 | 219 | 33 | |
| peak 4 | 198 | 231 | 16 | |
| peak 5 | — | — | — | |
| peak 6 | 222 | 294 | 122 | 240 |
| LFP/NCM | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于电极 产热/(J•g-1) |
|---|---|---|---|---|
| peak 1 | 130 | 180 | 84 | 1408 |
| peak 2 | 174 | 205 | 299 | |
| peak 3 | 213 | 228 | 140 | 2293 |
| peak 4 | 231 | 236 | 267 | |
| peak 5 | 241 | 245 | 29 | |
| peak 6 | 280 | 287 | 21 | 152 |
| NCM | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于电极 产热/(J•g-1) |
| peak 1 | 154 | 180 | 66 | 1632 |
| peak 2 | 185 | 208 | 378 | |
| peak 3 | 211 | 224 | 294 | 1285 |
| peak 4 | 232 | 235 | 109 | |
| peak 5 | 226 | 236 | 249 | |
| peak 6 | — | — | — | |
| LFP | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于电极 产热/(J•g-1) |
| peak 1 | 160 | 168 | 8 | 448 |
| peak 2 | 180 | 209 | 65 | |
| peak 3 | 197 | 219 | 33 | |
| peak 4 | 198 | 231 | 16 | |
| peak 5 | — | — | — | |
| peak 6 | 222 | 294 | 122 | 240 |

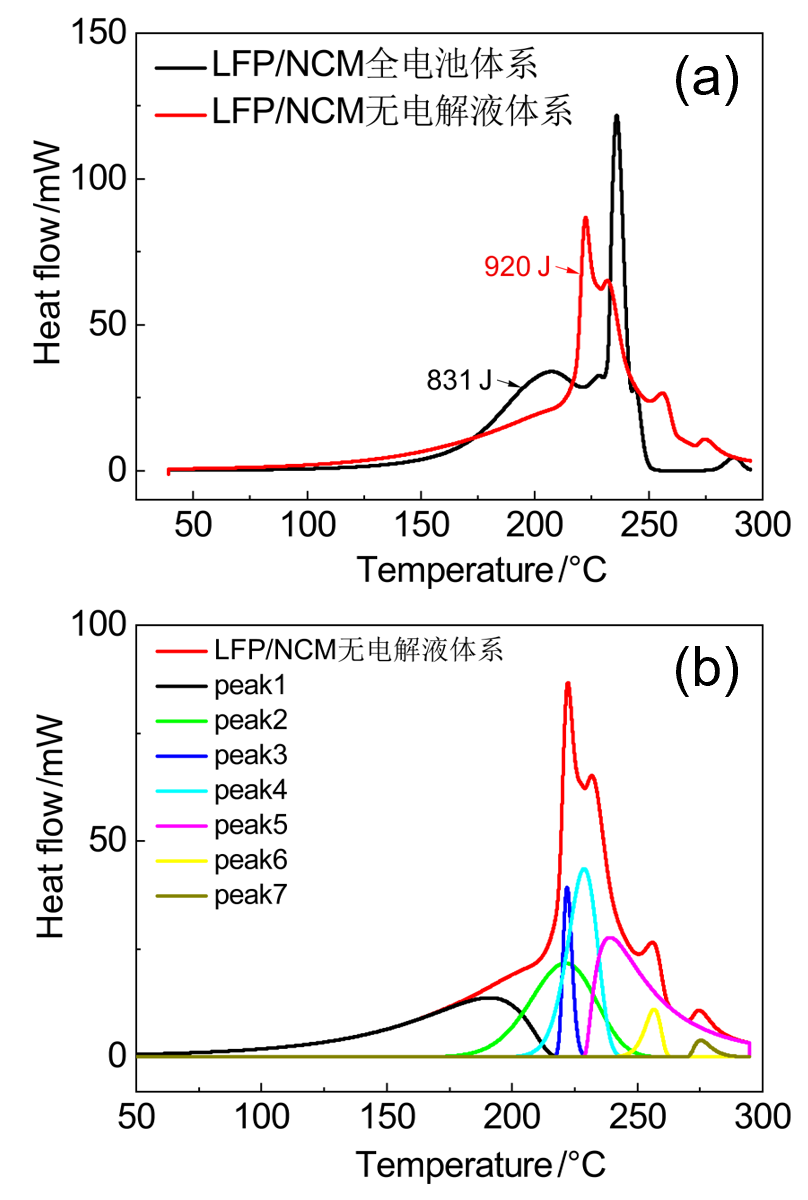
| LFP/NCM无电解液 | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于电极 产热/(J•g-1) |
|---|---|---|---|---|
| peak 1 | 138 | 194 | 161 | 592 |
| peak 2 | 193 | 221 | 211 | 4057 |
| peak 3 | 218 | 222 | 53 | |
| peak 4 | 215 | 228 | 197 | |
| peak 5 | 229 | 239 | 259 | |
| peak 6 | 248 | 256 | 27 | 125 |
| Peak7 | 270 | 275 | 14 |
| LFP/NCM无电解液 | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于电极 产热/(J•g-1) |
|---|---|---|---|---|
| peak 1 | 138 | 194 | 161 | 592 |
| peak 2 | 193 | 221 | 211 | 4057 |
| peak 3 | 218 | 222 | 53 | |
| peak 4 | 215 | 228 | 197 | |
| peak 5 | 229 | 239 | 259 | |
| peak 6 | 248 | 256 | 27 | 125 |
| Peak7 | 270 | 275 | 14 |
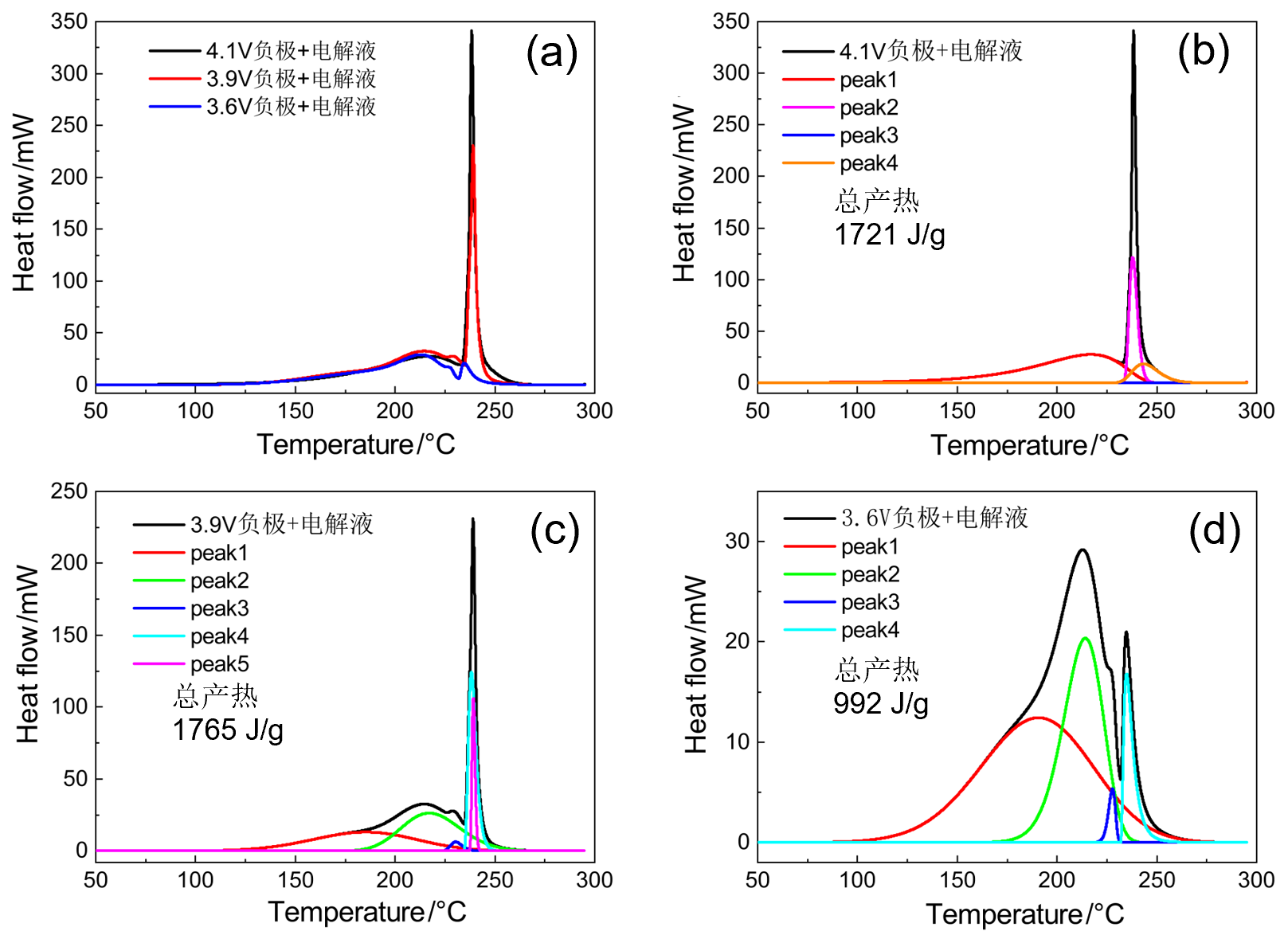
| 4.1V-SOC-90% | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于负极 产热/(J•g-1) |
|---|---|---|---|---|
| peak 1 | 160 | 218 | 845 | 1721 |
| peak 2 | — | — | — | |
| peak 3 | 226 | 229 | 271 | |
| peak 4 | 229 | 232 | 418 | |
| peak 5 | 234 | 243 | 187 | |
| 3.9V-SOC-70% | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于负极 产热/(J•g-1) |
| peak 1 | 130 | 185 | 567 | 1765 |
| peak 2 | 180 | 217 | 622 | |
| peak 3 | 225 | 230 | 30 | |
| peak 4 | 235 | 238 | 413 | |
| peak 5 | 237 | 239 | 133 | |
| 3.6V-SOC-30% | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于负极 产热/(J•g-1) |
| peak 1 | 110 | 190 | 571 | 992 |
| peak 2 | 170 | 214 | 336 | |
| peak 3 | 220 | 227 | 16 | |
| peak 4 | 232 | 234 | 69 | |
| peak 5 | — | — | — |
| 4.1V-SOC-90% | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于负极 产热/(J•g-1) |
|---|---|---|---|---|
| peak 1 | 160 | 218 | 845 | 1721 |
| peak 2 | — | — | — | |
| peak 3 | 226 | 229 | 271 | |
| peak 4 | 229 | 232 | 418 | |
| peak 5 | 234 | 243 | 187 | |
| 3.9V-SOC-70% | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于负极 产热/(J•g-1) |
| peak 1 | 130 | 185 | 567 | 1765 |
| peak 2 | 180 | 217 | 622 | |
| peak 3 | 225 | 230 | 30 | |
| peak 4 | 235 | 238 | 413 | |
| peak 5 | 237 | 239 | 133 | |
| 3.6V-SOC-30% | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于负极 产热/(J•g-1) |
| peak 1 | 110 | 190 | 571 | 992 |
| peak 2 | 170 | 214 | 336 | |
| peak 3 | 220 | 227 | 16 | |
| peak 4 | 232 | 234 | 69 | |
| peak 5 | — | — | — |
| 样品 | NCM全电池体系(mg) | LFP全电池 体系(mg) | 混合全电池体系(mg) | 负极+电解液体系(mg) |
|---|---|---|---|---|
| 负极极片 | 272 | 272 | 272 | 450 |
| 正极极片 | 507 | 507 | 507 | — |
| 隔膜 | 50 | 50 | 50 | — |
| 电解液 | 150 | 150 | 150 | 240 |
| m(负极)∶m(电解液) | 1.81 | 1.81 | 1.81 | 1.87 |
| 样品 | NCM全电池体系(mg) | LFP全电池 体系(mg) | 混合全电池体系(mg) | 负极+电解液体系(mg) |
|---|---|---|---|---|
| 负极极片 | 272 | 272 | 272 | 450 |
| 正极极片 | 507 | 507 | 507 | — |
| 隔膜 | 50 | 50 | 50 | — |
| 电解液 | 150 | 150 | 150 | 240 |
| m(负极)∶m(电解液) | 1.81 | 1.81 | 1.81 | 1.87 |
| [1] |
Li, M.; Lu, J.; Chen, Z.; Amine, K. Adv. Mater. 2018, 30, 1800561.
doi: 10.1002/adma.v30.33 |
| [2] |
Xu, J.; Cai, X.; Cai, S.; Shao, Y.; Hu, C.; Lu, S.; Ding, S. Energy Environ. Mater. 2023, 6, e12450.
doi: 10.1002/eem2.v6.5 |
| [3] |
Li, J.; Fleetwood, J.; Hawley, W. B.; Kays, W. Chem. Rev. 2022, 122, 903.
doi: 10.1021/acs.chemrev.1c00565 |
| [4] |
Yuan, Z.; Zhang, H.; Hu, S.; Zhang, B.; Zhang, J.; Cui, G. Acta Chim. Sinica 2023, 81, 1064. (in Chinese)
doi: 10.6023/A23030085 |
|
(苑志祥, 张浩, 胡思伽, 张波涛, 张建军, 崔光磊, 化学学报, 2023, 81, 1064.)
doi: 10.6023/A23030085 |
|
| [5] |
Yu, Q.; Nie, Y.; Peng, S.; Miao, Y.; Zhai, C.; Zhang, R.; Han, J.; Zhao, S.; Pecht, M. Appl. Energ. 2023, 349, 121674.
doi: 10.1016/j.apenergy.2023.121674 |
| [6] |
Cai, S.; Zhang, X.; Ji, J. J. Energy Storage 2023, 72, 108750.
doi: 10.1016/j.est.2023.108750 |
| [7] |
Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Energy Storage Mater. 2018, 10, 246.
|
| [8] |
Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. J. Power Sources 2012, 208, 210.
doi: 10.1016/j.jpowsour.2012.02.038 |
| [9] |
Xu, Z.; Zhou, X.; Fu, J.; Li, Q.; Tan, Z.; Fan, X.; Wang, Z.; Tian, B.; Guo, X. Chinese Sci. Bull. 2023, 68, 4501. (in Chinese)
doi: 10.1360/TB-2023-0273 |
|
(徐振恒, 周晓燕, 付佳龙, 李秋桐, 谭则杰, 樊小鹏, 王志明, 田兵, 郭新, 科学通报, 2023, 68, 4501.)
|
|
| [10] |
Zheng, Y.; Che, Y.; Hu, X.; Sui, X.; Stroe, D. I.; Teodorescu, R. Prog. Energ. Combust. 2024, 100, 101120.
doi: 10.1016/j.pecs.2023.101120 |
| [11] |
Khan, M. M.; Alkhedher, M.; Ramadan, M.; Ghazal, M. J. Energy Storage 2023, 73, 108775.
doi: 10.1016/j.est.2023.108775 |
| [12] |
Ma, S.; Jiang, M.; Tao, P.; Song, C.; Wu, J.; Wang, J.; Deng, T.; Shang, W. Prog. Nat. Sci. 2018, 28, 653.
doi: 10.1016/j.pnsc.2018.11.002 |
| [13] |
Ping, P.; Wang, Q.; Huang, P.; Sun, J.; Chen, C. Appl. Energ. 2014, 129, 261.
doi: 10.1016/j.apenergy.2014.04.092 |
| [14] |
Ping, P. Ph.D. Dissertation, University of Science and Technology of China, Hefei, 2014. (in Chinese)
|
|
(平平, 博士论文,中国科学技术大学, 合肥, 2014.)
|
|
| [15] |
Huang, P. F. Ph.D. Dissertation, University of Science and Technology of China, Hefei, 2018. (in Chinese)
|
|
(黄沛丰, 博士论文, 中国科学技术大学, 合肥, 2018.)
|
|
| [16] |
Feng, X.; Zheng, S.; Ren, D.; He, X.; Wang, L.; Cui, H.; Liu, X.; Jin, C.; Zhang, F.; Xue, C.; Hsub, H.; Gao, S.; Chen, T.; Li, Y.; Wang, T.; Wang, H.; Li, M.; Ouyang, M. Appl. Energ. 2019, 246, 53.
doi: 10.1016/j.apenergy.2019.04.009 |
| [17] |
Bak, S. M.; Hu, E. Y.; Zhou, Y. N.; Yu, X. Q.; Senanayake, S. D.; Cho, S. J.; Kim, K. B.; Chung, K. Y.; Yang, X. Q.; Nam, K. W. ACS Appl. Mater. Interfaces 2014, 6, 22594.
doi: 10.1021/am506712c |
| [18] |
Li, C.; Wang, H. W.; Han, X. B.; Wang, Y.; Wang, Y.; Zhang, Y. J.; Feng, X. N.; Ouyang, M. G. J. Electrochem. Energy. 2021, 8, 021012.
|
| [19] |
Mao, B. B.; Liu, C. Q.; Yang, K.; Li, S.; Liu, P. J.; Zhang, M. J.; Meng, X. D.; Gao, F.; Duan, Q. L.; Wang, Q. S.; Sun, J. H. Renew. Sust. Energ. Rev. 2021, 139, 110717.
doi: 10.1016/j.rser.2021.110717 |
| [20] |
Wang, Q. S.; Jiang, L. H.; Yu, Y.; Sun, J. H. Nano Energy 2019, 55, 93.
doi: 10.1016/j.nanoen.2018.10.035 |
| [21] |
Zhu, X.; Sun, Z.; Wang, Z.; Wang, H.; Lin, N.; Shan, C. J. Energy Storage 2023, 68, 107768.
doi: 10.1016/j.est.2023.107768 |
| [22] |
Liang, C.; Zhang, W. H.; Wei, Z. S.; Wang, Z. Y.; Wang, Q. S.; Sun, J. H. J. Energy Chem. 2021, 59, 446.
doi: 10.1016/j.jechem.2020.11.024 |
| [23] |
Peng, Y.; Yang, L. Z.; Ju, X. Y.; Liao, B. S.; Ye, K.; Li, L.; Cao, B.; Ni, Y. J. Hazard Mater. 2020, 381, 120916.
doi: 10.1016/j.jhazmat.2019.120916 |
| [24] |
Liang, C.; Jiang, L. H.; Ye, S. L.; Wang, Z. Y.; Wei, Z. S.; Wang, Q. S.; Sun, J. H. J. Energy Chem. 2021, 54, 332.
doi: 10.1016/j.jechem.2020.06.008 |
| [25] |
Duh, Y. S.; Lee, C. Y.; Chen, Y. L.; Kao, C. S. Thermochim. Acta 2016, 642, 88.
doi: 10.1016/j.tca.2016.09.007 |
| [26] |
Jiang, L. H.; Wang, Q. S.; Sun, J. H. J. Hazard Mater. 2018, 351, 260.
doi: 10.1016/j.jhazmat.2018.03.015 |
| [27] |
Duan, J.; Tang, X.; Dai, H. F.; Yang, Y.; Wu, W. Y.; Wei, X. Z.; Huang, Y. H. Electrochem. Energ. Rev. 2020, 3, 1.
doi: 10.1007/s41918-019-00060-4 |
| [1] | Jia Yanggang, Chen Shijie, Shao Xia, Cheng Jie, Lin Na, Fang Daolai, Mao Aiqin, Li Canhua. Preparation and High-performance Lithium-ion Storage of Cobalt-free Perovskite High-entropy Oxide Anode Materials [J]. Acta Chimica Sinica, 2023, 81(5): 486-495. |
| [2] | Yalan Zhang, Zhixiang Yuan, Hao Zhang, Jianjun Zhang, Guanglei Cui. Research Progress of High-energy-density Solid-state Lithium Ion Batteries Employing Ni-rich Ternary Cathodes [J]. Acta Chimica Sinica, 2023, 81(12): 1724-1738. |
| [3] | Wanying Chang, Yingying Tan, Jingyi Wu, Yingjie Liu, Jinhai Cai, Chunyan Lai. Study on the Properties of Polyethylene Oxide Based Solid State Electrolyte Enhanced by Three-Dimensional Structured Li6.28La3Zr2Al0.24O12 [J]. Acta Chimica Sinica, 2023, 81(12): 1708-1715. |
| [4] | Shuang Zhang, Chengfei Yang, Yubo Yang, Ningning Feng, Gang Yang. Electrochemical Behaviors of LixMO (x=0.79, 0.30, 0.08; M=Ni/Co/Mn) Recycled from Spent Li-ion Batteries as Cathodic Catalyst for Lithium-Oxygen Battery [J]. Acta Chimica Sinica, 2022, 80(9): 1269-1276. |
| [5] | Shouxiao Chen, Junke Liu, Weichen Zheng, Guozhen Wei, Yao Zhou, Juntao Li. Electron/ion Conductor Double-coated LiNi0.8Co0.1Mn0.1O2 Li-ion Battery Cathode Material and Its Electrochemical Performance [J]. Acta Chimica Sinica, 2022, 80(4): 485-493. |
| [6] | Kai Qiu, Mingxia Yan, Shouwang Zhao, Shengli An, Wei Wang, Guixiao Jia. Theoretical Study on the Structural Stability and Oxygen Ion Oxidation of Al-doped Lithium-ion Battery Layered Cathode Li(Li0.17Ni0.17Al0.04Fe0.13Mn0.49)O2 [J]. Acta Chimica Sinica, 2021, 79(9): 1146-1153. |
| [7] | Ping Xu, Xihua Zhang, En Ma, Fu Rao, Chunwei Liu, Peifan Yao, Zhi Sun, Jingwei Wang. Selective Recovery of Lithium from Spent Lithium-ion Batteries Synergized by Carbon and Sulfur Elements [J]. Acta Chimica Sinica, 2021, 79(8): 1073-1081. |
| [8] | Zhi Chang, Yu Qiao, Huijun Yang, Han Deng, Xingyu Zhu, Ping He, Haoshen Zhou. Applications of Metal-organic Frameworks (MOFs) Materials in Lithium-ion Battery/Lithium-metal Battery Electrolytes [J]. Acta Chimica Sinica, 2021, 79(2): 139-145. |
| [9] | Wang Shan, Fan Xiaoyong, Cui Yu, Gou Lei, Wang Xingang, Li Donglin. Three-dimensional Porous Current Collector for Lithium Storage Enhancement of NiO Electrode [J]. Acta Chim. Sinica, 2019, 77(6): 551-558. |
| [10] | Deng Bangwei, Sun Daming, Wan Qi, Wang Hao, Chen Tao, Li Xuan, Qu Meizhen, Peng Gongchang. Review of Electrolyte Additives for Ternary Cathode Lithium-ion Battery [J]. Acta Chim. Sinica, 2018, 76(4): 259-277. |
| [11] | He Qian, Zhang Chong, Li Xiao, Wang Xue, Mu Pan, Jiang Jiaxing. Pyrene-Based Conjugated Microporous Polymer as High Performance Electrode for Lithium-Ion Batteries [J]. Acta Chim. Sinica, 2018, 76(3): 202-208. |
| [12] | Li Zhiwei, Zhong Jialiang, Chen Nannan, Xue Bing, Mi Hongyu. Template-Assisted Preparation and Lithium Storage Performance of Nitrogen Doped Porous Carbon Sheets [J]. Acta Chim. Sinica, 2018, 76(3): 209-214. |
| [13] | Zheng Zhuo, Wu Zhenguo, Xiang Wei, Guo Xiaodong. Preparation and Electrochemical Performance of High Rate Spherical Layered LiNi0.5Co0.2Mn0.3O2 Cathode Material for Lithium-Ion Batteries [J]. Acta Chim. Sinica, 2017, 75(5): 501-507. |
| [14] | Zhang Guobin, Xiong Tengfei, Pan Xuelei, Yan Mengyu, Han Chunhua, Mai Liqiang. In Situ Observation and Mechanism Investigation of Lattice Breathing in Vanadium Oxide Cathode [J]. Acta Chim. Sinica, 2016, 74(7): 582-586. |
| [15] | Lyu Zhiyang, Feng Rui, Zhao Jin, Fan Hao, Xu Dan, Wu Qiang, Yang Lijun, Chen Qiang, Wang Xizhang, Hu Zheng. Nitrogen-Doped Carbon Nanocages as High-Rate Anode for Lithium Ion Batteries [J]. Acta Chim. Sinica, 2015, 73(10): 1013-1017. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||