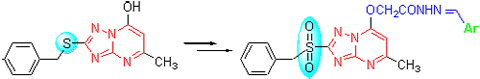
| [1] Chen, Q.; Zhu, X.-L.; Jiang, L.-L.; Liu, Z.-M.; Yang, G.-F. Eur. J. Med. Chem. 2008, 43, 595. [2] Chen, Q.; Liu, Z.-M.; Chen, C.-N.; Jiang, L.-L.; Yang, G.-F. Chem. Biodivers. 2009, 6, 1254. [3] Long, D.-Q.; Wang, Y.-G.; Li, D.-J.; Wang, F.-J. Chin. J. Org. Chem. 2005, 25, 1498 (in Chinese).(龙德清, 汪焱钢, 李德江, 王锋尖, 有机化学, 2005, 25, 1498.) [4] Tang, W.; Shi, D.-Q. J. Heterocycl. Chem. 2010, 47, 162. [5] Zhang, N.; Ayral-Kaloustian, S.; Nguyen, T.; Hernandez, R.; Beyer, C. Bioorg. Med. Chem. Lett. 2007, 17, 3003. [6] Zhai, X.; Jiang, N.; Zhang, K.-L.; Bao, F.; Gong, P. Chin. Chem. Lett. 2009, 20, 1179. [7] Linton, M. A.; Gonzalez, J.; Li, H.; Tatlock, J.; Jewell, T.; Johnson, S.; Drowns, M.; Patel, L.; Blazel, J.; Ornelas, M. Synthesis 2010, 4015. [8] Deev, S. L.; Yasko, M. V.; Karpenko, I. L.; Korovina, A. N.; Khandazhinskaya, A. L.; Andronova, V. L.; Galegov, G. A.; Shestakova, T. S.; Ulomskii, E. N.; Rusinov, V. L.; Chupakhin, O. N.; Kukhanova, M. K. Bioorg. Chem. 2010, 38, 265. [9] Yang, C.; Yang, S.; Song, B.-A.; Hu, D.-Y.; Chen, H.-J.; Xue, W.; Jin, L.-H.; Wu, J.; Xu, W.-M.; Bai, S. Chin. J. Org. Chem. 2010, 30, 1327 (in Chinese).(杨超, 杨松, 宋宝安, 胡德禹, 陈红军, 薛伟, 金林红, 吴剑, 徐维明, 柏松, 有机化学, 2010, 30, 1327.) [10] Li, Y.; Li, M.; Chai, B.-S.; Liu, C.-L. Agrochemicals 2006, 45, 697 (in Chinese).(李洋, 李淼, 柴宝山, 刘长令, 农药, 2006, 45, 697.) [11] Li, T.-G.; Liu, J.-P.; Han, J.-T.; Fu, B.; Wang, D.-Q.; Wang, M.-A. Chin. J. Org. Chem. 2009, 29, 898 (in Chinese).(李太公, 刘建平, 韩金涛, 傅滨, 王道全, 王明安, 有机化学, 2009, 29, 898.) [12] Wang, T.; Xue, S.-J. Chem. Reag. 2000, 22, 339 (in Chinese).(王涛, 薛思佳, 化学试剂, 2000, 22, 339.) [13] He, S.-Y.; Chen, J.-L.; Yang, R.; Wu, W.-T.; Zhao, J.-S.; Shi, Q.-Z.; Wang, R.-X. Chin. J. Org. Chem. 2003, 23, 1387 (in Chinese).(何水样, 陈军利, 杨锐, 武望婷, 赵建社, 史启祯, 王汝贤, 有机化学, 2003, 23, 1387.) [14] Zhou, Z.-Z.; Chen, Q.; Yang, G.-F. Chin. J. Org. Chem. 2008, 28, 1385 (in Chinese).(周中振, 陈琼, 杨光富, 有机化学, 2008, 28, 1385.) [15] Wei, T.-B.; Zhang, Z.-R.; Shi, H.-X.; Cui, W.-H.; Zhang, Y.-M. Chin. J. Org. Chem. 2008, 28, 145 (in Chinese).(魏太保, 张治仁, 师海雄, 崔文辉, 张有明, 有机化学, 2008, 28, 145.) [16] Yang, G.-F.; Liu, Z.-M.; Lu, A.-H.; Zhuang, N.-B. Acta Chim. Sinica 2001, 59, 594 (in Chinese).(杨光富, 刘祖明, 陆爱红, 庄农波, 化学学报, 2001, 59, 594.) [17] Chen, Q.; Long, D.-Q.; Cheng, J.; Li, J.; Liu, Z.-M.; Yang, G.-F. Chem. J. Chin. Univ. 2006, 27, 454 (in Chinese).(陈琼, 龙德清, 程靖, 李晶, 刘祖明, 杨光富, 高等学校化学学报, 2006, 27, 454.) [18] Yang, Z.-P.; Shan, S. Chin. J. Org. Chem. 2003, 23, 363 (in Chinese).(杨振平, 单尚, 有机化学, 2003, 23, 363.) [19] Gao, Y.-L.; Lin, X.-F.; Han, F.-F.; Bao, X.-P. Chin. J. Org. Chem. 2011, 31, 1648 (in Chinese).(高元磊, 林选福, 韩菲菲, 鲍小平, 有机化学, 2011, 31, 1648.) |