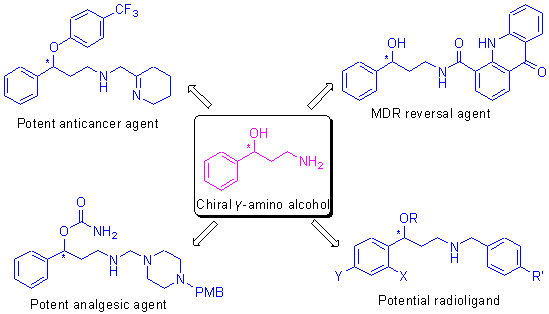
| [1] For selected papers, see: (a) Kelwala, S.; Stanley M.; Gershon, S.J.Clin.Psychiatry 1983, 44, 1.(b) Feighner, J.P.J.Clin.Psychiatry 1983, 44, 49.(c) Montgomery, S.A.J.Clin.Psychiatry 1985, 46, 3.(d) Rickels, K.; Smith, W.T.; Glaudin, V.; Amsterdam, J.B.; Weise, C.; Settle, G.P.J.Clin.Psychiatry 1985, 46, 38.(e) Romeo, E.; Strohle, A.; Spalletta, G.; di Michele, F.; Hermann, B.; Holsboer, F.; Pasini, A.; Rupprecht, R.Am.J.Psychiatry 1998, 155, 910.(f ) Kim, S.S.Ann.Pharmacother.2003, 37, 890.(g) Mowla, A.; Mosavinasab, M.; Pani, A.J.Clin.Psychopharmacol.2007, 27, 67.(h) Dharmshaktu, P.; Tayal, V.; Kalra, B.S.J.Clin.Pharmacol.2012, 52, 6.
[2] (a) Guarna, A.; Menchi, G.; Berti, G.; Cini, N.; Bottoncetti, A.; Raspanti, S.; Politib, A.Bioorg.Med.Chem.2001, 9, 3197.(b) Pupi, A.; Velingkar, V.S.; Dandekar, V.D.Chin.J.Chem.2011, 29, 504.(c) Chae, E.; Yi, H.; Choi, Y.; Cho, H.; Lee, K.; Moon, H.Bioorg.Med.Chem.Lett.2012, 22, 2434.(d) Spankuch, B.; Keppner, S.; Lange, L.; Rodrigues, T.; Zettl, H.; Koch, C.P.; Reutlinger, M.; Hartenfeller, M.; Schneider, P.; Schneider, G.Angew.Chem., Int.Ed.2013, 52, 4676.
[3] (a) Han, M.-L.; Hub, X.-P.; Huang, J.-D.; Chen, L.-G.; Zheng, Z.Tetrahedron: Asymmetry 2011, 22, 222.(b) Li, X.; Zhang, P.; Duan, K.; Wang, J.Chin.J.Org.Chem.2012, 32, 19.(c) Bera, P.K.; Ghosh, D.; Abdi, S.H.R.; Khan, N.H.J.Mol.Catal.Chem.2012, 361~362, 36.(d) Xie, J.; Zhou, Q.Acta Chim.Sinica 2012, 70, 1427.(e) Kumari, P.; Bera, P.K.; Khan, N.H.; Kureshy, R.I.; Abdi, S.H.R.; Bajaj, H.C.Catal.Sci.Technol.2014, 4, 563.
[4] For selected papers, see: (a) Gao Y.; Sharpless, K.B.J.Org.Chem.1988, 53, 4081.(b) Kitamura, M.; Ohkuma, T.; Inoue, S.; Sayo, N.; Kumobayashi, H.; Akutagawa, S.; Ohta, T.; Takaya, H.; Noyori, R.J.Am.Chem.Soc.1988, 110, 629.(c) Takeda, H.; Tachinami, T.; Aburatani, M.; Takahashi, H.; Morimoto, T.; Achiwa, K.Tetrahedron Lett.1989, 30, 363.(d) Sakuraba, S.; Achiwa, K.Synlett 1991, 689.(e) Takeda, H.; Hosokawa, S.; Aburatani, M.; Achiwa, K.Synlett 1991, 193.(f) Sakuraba, S.; Nakajima, N.; Achiwa, K.Synlett 1992, 829.(g) Sakuraba, S.; Nakajima, N.; Achiwa, K.Tetrahedron: Asymmetry 1993, 4, 1457.(h) Sakuraba, S.; Achiwa, K.Chem.Pharm.Bull.1995, 43, 748.(i) Ohkuma, T.; Koizumi, M.; Yoshida, M.; Noyori, R.Org.Lett.2000, 2, 1749.(j) Ohkuma, T.; Ishii, D.; Takeno, H.; Noyori, R.J.Am.Chem.Soc.2000, 122, 6510.(k) Ohkuma, T.; Koizumi, M.; Muniz, K.; Hilt, G.; Kabuto, C.; Noyori, R.J.Am.Chem.Soc.2002, 124, 6508.(l) Kamal, A.; Khanna G.B.R.; Ramu, R.Tetrahedron: Asymmetry 2002, 13, 2039.(m) Kakei, H.; Nemoto, T.; Ohshima, T.; Shibasaki, M.Angew.Chem., Int.Ed.2004, 43, 317.(n) Liu, D.; Gao, W.; Wang, C.; Zhang, X.Angew.Chem., Int.Ed.2005, 44, 1687.(o) Jing, Q.; Zhang, X.; Sun, J.; Ding, K.Adv.Synth.Catal.2005, 347, 1193.(p) Zhu, Q.; Shi, D.; Xia, C.; Huang, H.Chem.Eur.J.2011, 17, 7760.(q) Huang, H.; Zhu, Q.; Shi, D.; Xia, C.CN 102000606, 2011 [Abstr.Chem.2011, 154, 502603].(q) Geng, H.; Zhang, X.; Chang, M.; Zhou, L.; Wu, W.; Zhang, X.Adv.Synth.Catal.2011, 353, 3039.
[5] (a) Pàmies, O.; Bäckvall, J.-E.Adv.Synth.Catal.2002, 344, 947.(b) Watanabe, M.; Murata, K.; Ikariya, T.J.Org.Chem.2002, 67, 1712.(c) Kamal, A.; Khanna, G.B.R.; Ramu, R.Tetrahedron: Asymmetry 2002, 13, 2039.(d) Pandey, R.K.; Fernandes, R.A.; Kumar, P.Tetrahedron Lett.2002, 43, 4425.(e) Kumar, P.; Upadhyay R.K.; Pandey, R.K.Tetrahedron: Asymmetry 2004, 15, 3955.(f) Li, Y.; Li, Z.; Li, F.; Wang, Q.; Tao, F.Org.Biomol.Chem.2005, 3, 2513.
[6] (a) Lowe III, J.A.; Drozda, S.E.; Fisher, K.; Strick, C.; Lebel, L.; Schmidt, C.; Hiller D.; Zandi, K.S.Bioorg.Med.Chem.Lett.2003, 13, 1291.(b) Kamal, A.; Malik, M.S.; Shaik, A.A.; Azeeza, S.Tetrahedron: Asymmetry 2008, 19, 1078.
[7] (a) Wang, G.; Liu, X.; Zhao, G.Tetrahedron: Asymmetry 2005, 16, 1873.(b) Viswambharan, B.; Okimura, T.; Suzuki, S.; Okamoto, S.J.Org.Chem.2011, 76, 6678.
[8] Ghorai, M.K.; Das, K.; Shukla, D.J.Org.Chem.2007, 72, 5859.
[9] For reviews, see: (a) Li, Y.; Zheng, Y.; Tian, F.; Zhang, Y.-J.; Zhang, W.Chin.J.Org.Chem.2009, 29, 1487.(b) Zhang, W.; Liu, D.In Chiral Ferrocenes in Asymmetric Catalysis: Synthesis and Applications, Vol.14, Eds.: Dai, L.-X.; Hou, X.-L., VCH, Weinheim, Germany, 2010, p.175.(c) Butt, N.A.; Liu, D.; Zhang, W.Synlett 2014, 25, 615.(d) Zhang, W.; Adachi, Y.; Hirao T.; Ikeda, I.Tetrahedron: Asymmetry 1996, 7, 451.(e) Zhang, W.; Hirao, T.; Ikeda, I.Tetrahedron Lett.1996, 37, 4545.(f) Zhang, W.; Kida, T.; Nakatsuji, Y.; Ikeda, I.Tetrahedron Lett.1996, 37, 7995.(g) Zhang, W.; Yoneda, Y.-I.; Kida, T.; Nakatsuji, Y.; Ikeda, I.Tetrahedron: Asymmetry 1998, 9, 3371.(h) Zhang, W.; Yoneda, Y.-I.; Kida, T.; Nakatsuji, Y.; Ikeda, I.J.Organomet.Chem.1999, 574, 19.(i) Imai, Y.; Zhang, W.; Kida, T.; Nakatsuji, Y.; Ikeda, I.Chem.Lett.1999, 243.(j) Zhang, W.; Shimanuki, T.; Kida, T.; Nakatsuji, Y.; Ikeda, I.J.Org.Chem.1999, 64, 6247.(k) Zhang, W.; Yoshinaga, H.; Imai, Y.; Kida, T.; Nakatsuji, Y.; Ikeda, I.Synlett 2000, 1512.(l) Zhang, W.; Xie, F.; Yoshinaga, H.; Kida, T.; Nakatsuji, Y.; Ikeda, I.Tetrahedron 2006, 62, 9038.(m) Liu, D.; Xie, F.; Zhang, W.Tetrahedron Lett.2007, 48, 585.(n) Liu, D.; Xie, F.; Zhang, W.Tetrahedron Lett.2007, 48, 7591.(o) Liu, D.; Xie, F.; Zhang, W.J.Org.Chem.2007, 72, 6992.(p) Liu, D.; Xie, F.; Zhao, X.; Zhang, W.Tetrahedron 2008, 64, 3561.(q) Xie, F.; Liu, D.; Zhang, W.Tetrahedron Lett.2008, 49, 1012.(r) Wang, Y.; Liu, D.; Meng, Q.; Zhang, W.Tetrahedron: Asymmetry 2009, 20, 2510.(s) Zhao, X.; Liu, D.; Xie, F.; Zhang, W.Tetrahedron 2009, 65, 512.(t) Zhao, X.; Liu, D.; Xie, F.; Liu, Y.; Zhang, W.Org.Biomol.Chem.2011, 9, 1871.(u) Zhao, X.; Liu, D.; Guo, H.; Liu, Y.; Zhang, W.J.Am.Chem.Soc.2011, 133, 19354.(v) Guo, H.; Liu, D.; Butt, N.; Liu, Y.; Zhang, W.Tetrahedron 2012, 68, 3295.(w) Wang, J.; Liu, D.; Liu, Y.; Zhang, W.Org.Biomol.Chem.2013, 11, 3855.(x) Huo, X.; Quan, M.; Yang, G.; Zhao, X.; Liu, D.; Liu, Y.; Zhang, W.Org.Lett.2014, 16, 1570.
[10] Guarna, A.; Menchi, G.; Berti, G.; Cini, N.; Bottoncetti, A.; Raspanti, S.; Politi, A.; Pupi, A.Bioorg.Med.Chem.2001, 9, 3197.
[11] Haruhisa, S.; Takeshi, M.Bull.Chem.Soc.Jpn.1965, 38, 1293.
[12] Guarna, A.; Pupi, A.; Berti, G.; Bottoncetti, A.; Menchi, G.EP 972764, 2000 [Abstr.Chem.2000, 132, 93206]. |