有机化学 ›› 2021, Vol. 41 ›› Issue (1): 284-296.DOI: 10.6023/cjoc202008044 上一篇 下一篇
研究论文
收稿日期:2020-08-24
修回日期:2020-09-07
发布日期:2020-09-09
通讯作者:
温庭斌
作者简介:基金资助:
Xinyu Wanga, Qihuan Lia, Tingbin Wena,*( )
)
Received:2020-08-24
Revised:2020-09-07
Published:2020-09-09
Contact:
Tingbin Wen
Supported by:文章分享
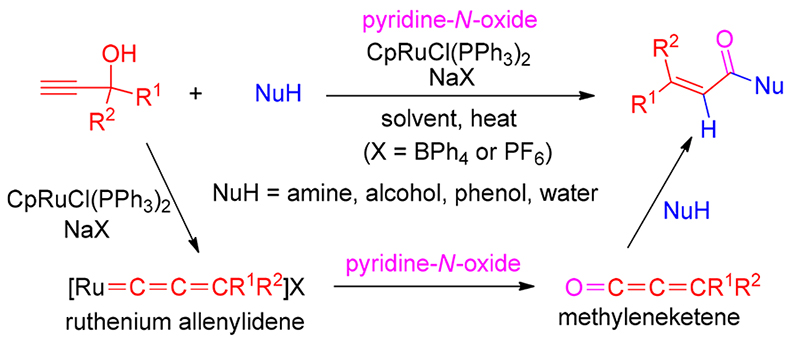
报道了钌催化末端炔丙醇经亚丙二烯基卡宾中间体氧化产生亚甲基烯酮合成 α, β-不饱和羧酸衍生物的高效方法. 机理研究实验表明, 催化剂CpRuCl(PPh3)2/NaBPh4和末端炔丙醇反应产生的钌亚丙二烯基卡宾与吡啶氧化物发生氧转移, 生成高活性的亚甲基烯酮中间体, 再发生亲核加成得到 α, β-不饱和产物. 该反应提供了一个机理上完全不同于传统方法的合成 α, β-不饱和羧酸衍生物的新策略, 是炔丙醇催化转化的一种新颖方法, 也是金属亚丙二烯基催化的一种新途径.
王新宇, 李其欢, 温庭斌. 钌催化末端炔丙醇经亚丙二烯基卡宾中间体氧化产生亚甲基烯酮: α, β-不饱和羧酸衍生物的合成[J]. 有机化学, 2021, 41(1): 284-296.
Xinyu Wang, Qihuan Li, Tingbin Wen. Ruthenium-Catalyzed Oxygenative Transformation of Terminal Propargyl Alcohols to Metheyleneketenes via Allenylidene Intermedia-tes: Synthesis ofα,β-Unsaturated Carboxylic Acid Derivatives[J]. Chinese Journal of Organic Chemistry, 2021, 41(1): 284-296.
| Entry | x/mol% | Addtive | Yield b /% |
|---|---|---|---|
| 1 | 10 | NaPF 6 (1 equiv.) | 95 |
| 2 | 10 | None | Trace |
| 3 | None | With or without NaPF 6 | 0 |
| 4 c | 10 | NaPF 6 (1 equiv.) | 84 |
| 5 | 10 | NaPF 6 (20 mol%) | 42 |
| 6 | 10 | NaBPh 4 (20 mol%) | 98 |
| 7 | 5 | NaBPh 4 (10 mol%) | 85 |
| 8 d | 5 | NaBPh 4 (10 mol%) | 97 |
| 9 d | 5 | NaBPh 4 (7.5 mol%) | 91 |
| Entry | x/mol% | Addtive | Yield b /% |
|---|---|---|---|
| 1 | 10 | NaPF 6 (1 equiv.) | 95 |
| 2 | 10 | None | Trace |
| 3 | None | With or without NaPF 6 | 0 |
| 4 c | 10 | NaPF 6 (1 equiv.) | 84 |
| 5 | 10 | NaPF 6 (20 mol%) | 42 |
| 6 | 10 | NaBPh 4 (20 mol%) | 98 |
| 7 | 5 | NaBPh 4 (10 mol%) | 85 |
| 8 d | 5 | NaBPh 4 (10 mol%) | 97 |
| 9 d | 5 | NaBPh 4 (7.5 mol%) | 91 |
| Entry | Catalyst | Additive | Yield b /% |
|---|---|---|---|
| 1 | CpRuCl(PPh 3) 2 | NaBPh 4 (10 mol%) | 97 |
| 3 c | [Ru( μ-Cl)(DPPQ) 2] 2- [BPh 4] 2 | None | Trace |
| 2 | CpRuCl(dppe) | NaBPh 4 (10 mol%) | 30 |
| 4 | Other Ru catalysts d | NaBPh 4 (10 mol%) | 0 |
| 5 e | RhCl(PPh 3) 3 | NaPF 6 (1 equiv.) or none | 0 |
| 6 e , f | [RhCl(COD)] 2 | P( p-FC 6H 4) 3 (30 mol%) NaPF 6 (1 equiv.) or none | 0 |
| Entry | Catalyst | Additive | Yield b /% |
|---|---|---|---|
| 1 | CpRuCl(PPh 3) 2 | NaBPh 4 (10 mol%) | 97 |
| 3 c | [Ru( μ-Cl)(DPPQ) 2] 2- [BPh 4] 2 | None | Trace |
| 2 | CpRuCl(dppe) | NaBPh 4 (10 mol%) | 30 |
| 4 | Other Ru catalysts d | NaBPh 4 (10 mol%) | 0 |
| 5 e | RhCl(PPh 3) 3 | NaPF 6 (1 equiv.) or none | 0 |
| 6 e , f | [RhCl(COD)] 2 | P( p-FC 6H 4) 3 (30 mol%) NaPF 6 (1 equiv.) or none | 0 |
| [1] |
For selected reviews on the organometallic properties of vinylidene complexes, see: (a) Bruce M. I. Chem. Rev. 1991, 91, 197.
|
|
(b) Puerta M.C.; Valerga P. Coord. Chem. Rev. 1999, 193 ~195, 977.
|
|
|
(c) Wakatsuki Y. J. Organomet. Chem. 2004, 689, 4092.
|
|
|
(d) Qiu Z.; Xie Z. Sci. China, Ser. B :Chem. 2009, 52, 1544.
|
|
|
(e) Lynam J.M. Chem. -Eur. J. 2010, 16, 8238.
|
|
|
(f) Herndon J.W. Coord. Chem. Rev. 2018, 356, 1.
|
|
| [2] |
For selected reviews on the organometallic properties of allenylidene complexes, see: (a) Bruce M. I. Chem. Rev. 1998, 98, 2797.
|
|
(b) Selegue J.P. Coord. Chem. Rev. 2004, 248, 1543.
|
|
|
(c) Rigaut S.; Touchard D.; Dixneuf P.H. Coord. Chem. Rev. 2004, 248, 1585.
|
|
|
(d) Che C.; Ho C.; Huang J. Coord. Chem. Rev. 2007, 251, 2145.
|
|
|
(e) Cadierno V.; Gimeno J. Chem. Rev. 2009, 109, 3512.
|
|
|
(f) Herndon J.W. Coord. Chem. Rev. 2019, 401, 213051.
|
|
| [3] |
For selected reviews on metal vinylidenes and allenylidenes in catalysis, see: (a) Bruneau C.; Dixneuf P. H. Acc. Chem. Res. 1999, 32, 311.
|
|
(b) Trost B.M.; Toste F.D.; Pinkerton A.B. Chem. Rev. 2001, 101, 2067.
|
|
|
(c) Miki K.; Uemura S.; Ohe K. Chem. Lett. 2005, 34, 1068.
|
|
|
(d) Varela J.A.; Saá C. Chem. -Eur. J. 2006, 12, 6450.
|
|
|
(e) Trost B.M.; McClory A. Chem. -Asian J. 2008, 3, 164.
|
|
|
Bruneau C.; Dixneuf P.H. Metal Vinylidenes and Allenylidenes in Catalysis :From Reactivity to Applications in Synthesis, WILEY-VCH, Weinheim, Germany, 2008.
|
|
|
Varela J.A.; González-Rodríguez C.; Saá C. Ruthenium in Catalysis, InTopics in Organometallic Chemistry Series 48, Eds.: Bruneau, C.; Dixneuf, P.H., Springer, Switzerland, 2014, pp.237~288.
|
|
|
(h) Roh S.W.; Choi K.; Lee C. Chem. Rev. 2019, 119, 4293.
|
|
|
Gagosz F. Synthesis -Stuttgart 2019, 51, 1087.
|
|
|
(j) Jin J.-T.; Tao X.-C.; Qian Y.-L. Chin. J. Org. Chem. 2000, 20, 470. (in Chinese)
|
|
|
( 金军挺, 陶晓春, 钱延龙, 有机化学, 2000, 20, 470.).
|
|
| [4] |
Coletti C.; Marrone A.; Re N. Acc. Chem. Res. 20 12, 45, 139.
|
| [5] |
(a) Hyder I.; Jiménez-Tenorio M.; Puerta M.C.; Valerga P. Organometallics 2011, 30, 726.
|
|
(b) Talavera M.; Bolaño S.; Bravo J.; Castro J.; Garcı́a-Fontán S.; Hermida-Ramón J.M. Organometallics 2013, 32, 4402.
|
|
|
(c) Serrano-Ruiz M.; Lidrissi C.; Mañas S.; Peruzzini M.; Romerosa A. J. Organomet. Chem. 2014, 751, 654.
|
|
|
(d) Jiménez-Tenorio M.; Puerta M.C.; Valerga P. Organometallics 2016, 35, 388 and references therein.
|
|
| [6] |
(a) Cadierno V.; Gamasa M.P.; Gimeno J.; Perez-Carreno E.; Ienco A. Organometallics 1998, 17, 5216.
|
|
(b) Esteruelas M.A.; Gomez A.V.; Lopez A.M.; Onate E.; Ruiz N. Organometallics 1999, 18, 1606.
|
|
|
(c) Cadierno V.; Conejero S.; Gamasa M.P.; Gimeno J.; Falvello L.R.; Llusar R.M. Organometallics 2002, 21, 3716.
|
|
|
(d) Saget T.; Cramer N. Angew. Chem., Int. Ed. 2010, 49, 8962.
|
|
|
(e) Queensen M.J.; Rath N.P.; Bauer E.B. Organometallics 2014, 33, 5052.
|
|
|
(f) García-de la Arada, I.; Díez, J.; Gamasa, M.P.; Lastra, E. J. Organomet. Chem. 2015, 797, 101.
|
|
| [7] |
(a) Bustelo E.; Jimenez-Tenorio M.; Puerta M.C.; Valerga P. Organometallics 2006, 25, 4019.
|
|
(b) Pino-Chamorro J.A.; Bustelo E.; Puerta M.C.; Valerga P. Organometallics 2009, 28, 1546.
|
|
| [8] |
(a) Trost B.M.; Frederiksen M.U.; Rudd M.T. Angew. Chem., Int. Ed. 2005, 44, 6630.
|
|
(b) Bruneau C.; Dixneuf P.H. Angew. Chem., Int. Ed. 2006, 45, 2176.
|
|
|
(c) Liu R.-S. Synlett 2008, 801.
|
|
| [9] |
(a) Nishibayashi Y.; Uemura S. Curr. Org. Chem 2006, 10, 135.
|
|
Nishibayashi Y. Synthesis 2012, 489.
|
|
|
(c) Sakata K.; Nishibayashi Y. Catal. Sci. Technol. 2018, 8, 12.
|
|
| [10] |
Zhang D.-Y.; Hu X.-P. Tetrahedron Lett. 2015, 56, 283.
|
| [11] |
Trost B.M.; Flygare J.A. J. Am. Chem. Soc. 1992, 114, 5476.
|
| [12] |
(a) Bustelo E.; Dixneuf P.H. Adv. Synth. Catal. 2005, 347, 393.
|
|
(b) Ma H.W.; Lin Y.C.; Huang S.L. Org. Lett. 2012, 14, 3846.
|
|
| [13] |
(a) Yeh K.L.; Liu B.; Lo C.Y.; Huang H.L.; Liu R.S. J. Am. Chem. Soc. 2002, 124, 6510.
|
|
(b) Yeh K.L.; Liu B.; Lai Y.T.; Li C.W.; Liu R.S. J. Org. Chem. 2004, 69, 4692.
|
|
|
(c) Shen H.C.; Su H.L.; Hsueh Y.C.; Liu R.S. Organometallics 2004, 23, 4332.
|
|
|
Propargylic reduction of propargylic alcohols with 2-Propanol via similar hydrogen transfer was reported:.
|
|
|
(d) Yuki M.; Miyake Y.; Nishibayashi Y. Organometallics 2010, 29, 5994.
|
|
| [14] |
Datta S.; Chang C.L.; Yeh K.L.; Liu R.S. J. Am. Chem. Soc. 2003, 125, 9294.
|
| [15] |
(a) Cadierno V.; Díez J.; García-Garrido S.E.; Gimeno J. Chem. Commun. 2004, 2716.
|
|
(b) Cadierno V.; Díez J.; García-Garrido S.E.; Gimeno J.; Nebra N. Adv. Synth. Catal. 2006, 348, 2125.
|
|
|
(c) Cadierno V.; García-Garrido S.E.; Gimeno J. Adv. Synth. Catal. 2006, 348, 101.
|
|
|
(d) Onodera G.; Matsumoto H.; Nishibayashi Y.; Uemura Y. Organometallics 2005, 24, 5799.
|
|
| [16] |
(a) Tidwell T.T. Angew. Chem., Int. Ed. 2005, 44, 5778.
|
|
(b) Allen A.D.; Tidwell T.T. Eur. J. Org. Chem. 2012, 2012, 1081.
|
|
|
(c) Allen A.D.; Tidwell T.T. Chem. Rev. 2013, 113, 7287.
|
|
| [17] |
(a) Madhushaw R.J.; Lin M.Y.; Abu Sohel S.M.; Liu R.S. J. Am. Chem. Soc. 2004, 126, 6895.
|
|
(b) Lin M.Y.; Madhushaw R.J.; Liu R.S. J. Org. Chem. 2004, 69, 7700.
|
|
|
(c) Lin M.Y.; Maddirala S.J.; Liu R.S. Org. Lett. 2005, 7, 1745.
|
|
|
(d) Pati K.; Liu R.S. Chem. Commun. 2009, 5233.
|
|
|
(e) Kim I.; Lee C. Angew. Chem., Int. Ed. 2013, 52, 10023.
|
|
|
(f) Kim I.; Roh S.W.; Lee D.G.; Lee C. Org. Lett. 2014, 16, 2482.
|
|
|
(g) Wang Y.; Zheng Z.; Zhang L. Angew. Chem., Int. Ed. 2014, 53, 9572.
|
|
|
(h) Zheng R.; Wang Y.; Zhang L. Tetrahedron Lett. 2015, 56, 3144.
|
|
|
(i) Zeng H.; Li C.J. Angew. Chem., Int. Ed. 2014, 53, 13862.
|
|
|
(j) Yu C.; Ma X.; Chen B.; Tang B.; Paton R.S.; Zhang G. Eur. J. Org. Chem. 2017, 2017, 1561.
|
|
|
(k) Rong M.G.; Qin T.Z.; Liu X.R.; Wang H.F.; Zi W. Org. Lett. 2018, 20, 6289.
|
|
|
(l) Zhang W.W.; Gao T.T.; Xu L.J.; Li B.J. Org. Lett. 2018, 20, 6534.
|
|
|
(m) Álvarez-Pérez A.; Esteruelas M.A.; Izquierdo S.; Varela J.A.; Saá C. Org. Lett. 2019, 21, 5346.
|
|
|
For a recent review, see:.
|
|
|
Álvarez-Pérez A.; Varela J.A.; Saá C. Synthesis 2020, 52, 2639.
|
|
| [18] |
Brown R.F.C.; Eastwood F.W. The Chemistry of Ketenes, Allenes and Related Compounds, John Wiley& Sons Ltd, New York , 1980, Chapter 19.
|
| [19] |
(a) Hart H.; Dean D.L.; Buchanan D.N. J. Am. Chem. Soc. 1973, 95, 6294.
|
|
(b) Chapman O.L.; Chang C.-C.; Hole J.; Rosenquist N.R.; Tomioka H. J. Am. Chem. Soc. 1975, 97, 22, 6586.
|
|
|
(c) Meng J.B.; Shen M.Q.; Wang X.H.; Gao Z.H.; Wang H.G.; Matsuura T. Chin. Sci. Bull. 1991, 36, 2056.
|
|
|
(d) Pietri N.; Monnier M.; Aycard J.P. J. Org. Chem. 1998, 63, 2462.
|
|
|
(e) Yang C.; Wu W.; Liu K.; Wang H.; Su H. Sci. China :Chem. 2012, 55, 359.
|
|
| [20] |
(a) Brown R.F.C.; Jones C.M. Aust. J. Chem. 1980, 33, 1817.
|
|
(b) Brown R.F.C.; Eastwood F.W.; Chaichit N.; Gatehouse B.M.; Pfeiffer J.M.; Woodroffe D. Aust. J. Chem. 1981, 34, 1467.
|
|
|
(c) Besida J.; Brown R.F.C. Aust. J. Chem. 1982, 35, 1385.
|
|
|
(d) Besida J.; Brown R.F.C.; Colmanet S.; Leach D.N. Aust. J. Chem. 1982, 35, 1373.
|
|
|
(e) Pommelet J.C.; Dhimane H.; Chuche J.; Celerier J.P.; Haddad M.; Lhommet G. J. Org. Chem. 1988, 53, 5680.
|
|
|
(f) Wentrup C.; Lorencak P. J. Am. Chem. Soc. 1988, 110, 1880.
|
|
|
(g) Brahms J.C.; Dailey W.P. J. Am. Chem. Soc. 1989, 111, 8940.
|
|
|
(h) Bencheikh A.; Pommelet J.C.; Chuche J. J. Chem. Soc., Chem. Commun. 1990, 615.
|
|
|
(i) Chuburu F.; Lacombe S.; Pfisterguillouzo G.; Bencheik A.; Chuche J.; Pommelet J.C. J. Am. Chem. Soc. 1991, 113, 1954.
|
|
|
(j) Fulloon B.E.; Wentrup C. J. Org. Chem. 1996, 61, 1363.
|
|
|
Gaber A.A.M.; McNab H. Synthesis -Stuttgart 2001, 2059.
|
|
|
(l) Halton B.; Dixon G.M.; Jones C.S.; Parkin C.T.; Veedu R.N.; Bornemann H.; Wentrup C. Org. Lett. 2005, 7, 949.
|
|
|
(m) Andersen H.G.; Wentrup C. Aust. J. Chem. 2012, 65 .
|
|
| [21] |
(a) Birum G.H.; Matthews C.N. J. Am. Chem. Soc. 1968, 90, 14, 3842.
|
|
(b) Taylor G.A. Chem. Commun. (London )1968, 1314.
|
|
|
(c) Taylor G.A. J. Chem. Soc. 1969, 1755.
|
|
|
(c) Masters A.P.; Sorensen T.S.; Tran P.M. Can. J. Chem. 1987, 65, 1499.
|
|
| [22] |
Cai T.; Yang Y.; Zhang L.; Wen T. Chin. J. Org. Chem. 2018, 38, 2017. (in Chinese)
|
|
( 蔡涛, 杨玉, 张丽, 温庭斌, 有机化学, 2018, 38, 2017.).
|
|
| [23] |
For catalytic nitrogen tranfer to metal vinylidenes with hydrazines for nitrile synthesis, see: (a) Fukumoto Y.; Dohi T.; Masaoka H.; Chatani N.; Murai S. Organometallics 2002, 21, 3845.
|
|
(b) Fukumoto Y.; Tamura Y.; Iyori Y.; Chatani N. J. Org. Chem. 2016, 81, 3161.
|
|
|
For stoichiometrc nitrogen tranfer to metal vinylidenes with hydrazines to give nitrile complexes, see: (c) Alt H.G.; Engelhardt H.E.; Steinlein E.; Rogers D. J. Organomet. Chem. 1987, 344, 321.
|
|
|
(d) Barrett A.G.M.; Carpenter N.E.; Sabat M. J. Organomet. Chem. 1988, 352, C8.
|
|
|
(e) Albertin G.; Antoniutti S.; Bortoluzzi M.; Botter A.; Castro J. Dalton Trans. 201 5, 44, 3439.
|
|
| [24] |
(a) Arshad L.; Jantan I.; Bukhari S.N.; Haque M.A. Front Pharmacol 2017, 8, 22.
|
|
(b) Hossain M.; Das U.; Dimmock J.R. Eur. J. Med. Chem. 2019, 183, 111687.
|
|
|
(c) Zhang S.; Neumann H.; Beller M. Chem. Soc. Rev. 2020, 49, 3187.
|
|
| [25] |
(a) Reichl K.D.; Dunn N.L.; Fastuca N.J.; Radosevich A.T. J. Am. Chem. Soc. 2015, 137, 5292.
|
|
(b) Meng L.K.; Kamada Y.; Muto K.; Yamaguchi J.; Itami K. Angew. Chem., Int. Ed. 2013, 52, 10048.
|
|
|
(c) Liu L.; Lu H.; Wang H.; Yang C.; Zhang X.; Zhang-Negrerie D.; Du Y.F.; Zhao K. Org. Lett. 2013, 15, 2906.
|
|
|
(d) Li Y.J.; Yang Q.; Yang L.Q.; Lei N.; Zheng K. Chem. Commun. 2019, 55, 4981.
|
|
| [26] |
Bruce M.I.; Low P.J.; Tiekink E.R.T. J. Organomet. Chem. 1999, 572, 3.
|
| [27] |
Following Saá’s conditions for the oxidative amidation of alkynes(see Ref.[17m]),1 equiv. of NaPF6 was used. The PF6 – anion is prone to dissociate into PF5 and F– at elevated temperature and the residual water presented in the solution may cause further hydrolysis of the resulting PF5 species to form phosphate. See: (a) Odedra A.; Datta S.; Liu R. S. J. Org. Chem. 2007, 72, 3289.
|
|
(b) Krossing I.; Raabe I. Chem. -Eur. J. 2004, 10, 5017.
|
|
|
(c) Krossing I.; Raabe I. Angew. Chem., Int. Ed. 2004, 43, 2066.
|
|
| [28] |
The DCE solvent may undergo dissociation to eliminate hydrogen chloride after prolonged heating. See: (a) Ho, M. L.; Flynn, A. B.; Ogilvie, W. W.J. Org. Chem. 2007, 72, 977.
|
|
(b) He W.; Xie L.; Xu Y.; Xiang J.; Zhang L. Org. Biomol. Chem. 2012, 10, 3168.
|
|
|
(c) Qian G.; Hong X.; Liu B.; Mao H.; Xu B. Org. Lett. 2014, 16, 5294.
|
|
| [29] |
(a) Pavlik S.; Mereiter K.; Puchberger M.; Kirchner K. J. Organomet. Chem. 2005, 690, 5497.
|
|
(b) Hartmann S.; Winter R.F.; Brunner B.M.; Sarkar B.; Knodler A.; Hartenbach I. Eur. J. Inorg. Chem. 2003, 876.
|
|
|
(c) Chan W.C.; Lau C.P.; Chen Y.Z.; Fang Y.Q.; Ng S.M.; Jia G.C. Organometallics 1997, 16, 34.
|
|
|
(d) Buriez B.; Burns I.D.; Hill A.F.; White A.J.P.; Williams D.J.; Wilton-Ely J.D.E. T. Organometallics 1999, 18, 1504.
|
|
| [30] |
(a) Picquet M.; Bruneau C.; Dixneuf P.H. Chem. Commun. 1998, 2249.
|
|
(b) Furstner A.; Liebl M.; Lehmann C.W.; Picquet M.; Kunz R.; Bruneau C.; Touchard D.; Dixneuf P.H. Chem. -Eur. J. 2000, 6, 1847.
|
|
| [31] |
Selegue J.P. J. Am. Chem. Soc. 1983, 105, 5921.
|
| [32] |
(a) Lukehart C.M.; Zelie J.V. J. Organomet. Chem. 1975, 97, 421.
|
|
(b) Wulff W.D.; Yang D.C. J. Am. Chem. Soc. 1983, 105, 6726.
|
|
|
(c) Dötz K.H. Angew. Chem., Int. Ed. 1984, 23, 587.
|
|
|
(d) Barrett A.G.M.; Mortier J.; Sabat M.; Sturgess M.A. Organometallics 1988, 7, 2553.
|
|
|
(e) Gibert M.; Ferrer M.; Lluch A.M.; Sánchez-Baeza F.; Messeguer A. J. Org. Chem. 1999, 64, 1591.
|
|
|
(f) Ruan W.; Shi C.; Sung H.H.Y.; Williams I.D.; Jia G. J. Organomet. Chem. 2019, 880, 7.
|
|
| [33] |
(a) Trost B.M.; Rhee Y.H. J. Am. Chem. Soc. 1999, 121, 11680.
|
|
(b) Trost B.M.; Rhee Y.H. J. Am. Chem. Soc. 2002, 124, 2528.
|
|
|
(c) Taduri B.P.; Sohel S.M.A.; Cheng H.M.; Lin G.Y.; Liu R.S. Chem. Commun. 2007, 2, 2530.
|
|
| [34] |
Bruce M.I.; Hameister C.; Swincer A.G.; Wallis R.C. Inorg. Synth. 1990, 28, 270.
|
| [35] |
Gluyas J.B.G.; Brown N.J.; Farmer J.D.; Low P.J. Aust. J. Chem. 2017, 70, 113.
|
| [36] |
Cai T.; Yang Y.; Li W.W.; Qin W.B.; Wen T.B. Chem. -Eur. J. 2018, 24, 1606.
|
| [37] |
Alcock N.W.; Burns I.D.; Claire K.S.; Hill A.F. Inorg. Chem. 1992, 31, 2906.
|
| [38] |
Boren B.C.; Narayan S.; Rasmussen L.K.; Zhang L.; Zhao H.T.; Lin Z.Y.; Jia G.C.; Fokin V.V. J. Am. Chem. Soc. 200 8, 130, 14900.
|
| [39] |
(a) Harada S.; Yano H.; Obora Y. ChemCatChem 2013, 5, 121.
|
|
(b) Han Y.P.; Song X.R.; Qiu Y.F.; Hao X.H.; Wang J.; Wu X.X.; Liu X.Y.; Liang Y.M. J. Org. Chem. 2015, 80, 9200.
|
|
|
(c) Groundwater P.W.; Garnett I.; Morton A.J.; Sharif T.; Coles S.J.; Hursthouse M.B.; Nyerges M.; Anderson R.J.; Bendell D.; McKillop A.; Zhang W. J. Chem. Soc., Perkin Trans. 1 2001, 2781.
|
|
|
(d) Ueda S.; Okada T.; Nagasawa H. Chem. Commun. 2010, 46, 2462.
|
|
|
(e) Reeves D.C.; Rodriguez S.; Lee H.; Haddad N.; Krishnamurthy D.; Senanayake C.H. Org. Lett. 2011, 13, 2495.
|
|
|
(f) Song C.E.; Jung D.U.; Choung S.Y.; Roh E.J.; Lee S.G. Angew. Chem., Int. Ed. 2 004, 43, 6183.
|
|
|
(g) Inamoto K.; Okawa H.; Taneda H.; Sato M.; Hirono Y.; Yonemoto M.; Kikkawa S.; Kondo Y. Chem. Commun. 2012, 48, 9771.
|
|
|
(h) Ito Y. Tetrahedron 2007, 63, 3108.
|
| [1] | 田雁, 董睿, 聂鹏, 许波. 含不同取代基的钌-锗化合物的合成及表征[J]. 有机化学, 2024, 44(1): 173-179. |
| [2] | 唐菁, 罗文坤, 周俊. 氮杂螺[4.5]三烯酮衍生物的合成研究进展[J]. 有机化学, 2023, 43(9): 3006-3034. |
| [3] | 张素珍, 张文文, 杨慧, 顾庆, 游书力. 铑催化2-烯基苯酚与炔烃的对映体选择性螺环化反应[J]. 有机化学, 2023, 43(8): 2926-2933. |
| [4] | 周章涛, 王杨, 程冰心, 叶伟平. [RuCl(p-cymene)-(S)-BINAP]Cl催化不对称合成反式-3-氨基-双环[2.2.2]辛烷-2-甲酸乙酯[J]. 有机化学, 2023, 43(8): 2961-2967. |
| [5] | 朱玥, 陈璐, 赵静, 孙庆荣, 杨维清, 付海燕, 马梦林. 取代8-羟基喹啉钌络合物催化Friedländer反应合成喹啉衍生物[J]. 有机化学, 2023, 43(7): 2528-2542. |
| [6] | 南江, 黄冠杰, 胡岩, 王波. 钌催化喹唑啉酮与碳酸亚乙烯酯的C—H [4+2]环化反应[J]. 有机化学, 2023, 43(4): 1537-1549. |
| [7] | 王维, 张哲宇, 张雪, 于海丰, 罗辉, 霍东月, 徐玉澎, 赵晓波. 多取代2,3-二氢-4-吡啶酮的水相合成[J]. 有机化学, 2023, 43(2): 742-750. |
| [8] | 刘鹏, 钟富明, 廖礼豪, 谭伟强, 赵晓丹. 炔烃参与的去芳构化反应构建螺环己二烯酮类化合物的研究进展[J]. 有机化学, 2023, 43(12): 4019-4035. |
| [9] | 赵晓正, 凌琴琴, 曹桂妍, 火星, 赵小龙, 苏瀛鹏. 炔丙醇类化合物参与的环化反应研究进展[J]. 有机化学, 2022, 42(9): 2605-2639. |
| [10] | 王梅, 龚慧华, 付海燕, 郑学丽, 陈华, 李瑞祥. 金属钌配合物催化醇与腈串联反应合成α-取代酰胺[J]. 有机化学, 2022, 42(8): 2418-2427. |
| [11] | 代增进, 张绪穆, 殷勤. 铵盐为胺源的不对称还原胺化反应研究进展[J]. 有机化学, 2022, 42(8): 2261-2274. |
| [12] | 郭泽, 吴迪, 王丽丽, 段征. BF3•Et2O促进的双烯酮-酚重排合成具有聚集诱导发光(AIE)效应的磷杂七元环化合物[J]. 有机化学, 2022, 42(8): 2481-2487. |
| [13] | 闫法超, 李洋, 李玉东, Mohamed Makha, 李跃辉. 氰甲基导向的吲哚选择性C—H烯基化[J]. 有机化学, 2022, 42(7): 2192-2200. |
| [14] | 李涛, 刘艺, 白雪, 周遵军, 左鹏, 麻妙锋, 仲崇民, 左亚杰. 咪唑离子官能化的HG-II型手性钌催化剂的制备及其催化的不对称烯烃复分解反应[J]. 有机化学, 2022, 42(6): 1713-1721. |
| [15] | 方霄龙, 李斌, 金杰, 段宁. 均相催化丙二酸二甲酯加氢制1,3-丙二醇的研究[J]. 有机化学, 2022, 42(5): 1407-1413. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||