有机化学 ›› 2021, Vol. 41 ›› Issue (4): 1691-1702.DOI: 10.6023/cjoc202010023 上一篇 下一篇
研究论文
收稿日期:2020-10-16
修回日期:2020-11-11
发布日期:2020-12-05
通讯作者:
程凯, 黄乐浩
基金资助:
Honglei Jina, Fengxuan Jiangb, Kai Chengb,*( ), Lehao Huanga,*(
), Lehao Huanga,*( )
)
Received:2020-10-16
Revised:2020-11-11
Published:2020-12-05
Contact:
Kai Cheng, Lehao Huang
About author:Supported by:文章分享
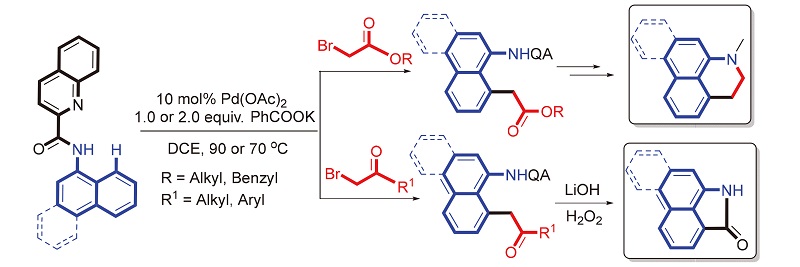
报道了一种钯催化1-萘酰胺的8位碳氢键烷基化反应. 在该反应中,喹啉甲酰胺作为N,N-双齿螯合基团, 各种取代的α-溴乙酸烷基酯以及α-溴代苯乙酮作为烷基化试剂, 高效、高区域选择性地合成了8-烷基-1-萘胺衍生物. 最后, 将含酯基和酮基的烷基化产物分别通过相应的衍生化反应合成具有多种生物活性的阿朴菲和马兜铃内酰胺类生物碱结构单元.
金红蕾, 姜风轩, 程凯, 黄乐浩. 钯催化1-萘酰胺的8-烷基化反应及其在阿朴菲和马兜铃内酰胺类生物碱骨架合成中的应用[J]. 有机化学, 2021, 41(4): 1691-1702.
Honglei Jin, Fengxuan Jiang, Kai Cheng, Lehao Huang. Palladium-Catalyzed C8 Alkylation of 1-Naphthylamides and Its Application to the Synthesis of the Core Sturctures of Aporphine and Aristolactam Alkaloids[J]. Chinese Journal of Organic Chemistry, 2021, 41(4): 1691-1702.
| Entry | Catalyst/mol% | Additive | Solvent | Yieldb/% |
|---|---|---|---|---|
| 1 | Pd(OAc)2 | KOAc | 1,4-Dioxane | 61 |
| 2 | Pd(OAc)2 | PhCOOK | 1,4-Dioxane | 68 |
| 3 | Pd(OAc)2 | PhCOOK | DMF | N.D. |
| 4 | Pd(OAc)2 | PhCOOK | Xylene | 82 |
| 5 | Pd(OAc)2 | PhCOOK | DMSO | N.D. |
| 6 | Pd(OAc)2 | PhCOOK | t-BuOH | 75 |
| 7 | Pd(OAc)2 | PhCOOK | DCE | 86 |
| 8 | Pd(OAc)2 | — | DCE | Trace |
| 9 | Pd(OAc)2 | AgOAc | DCE | Trace |
| 10 | Pd(OAc)2 | NaOAc | DCE | 49 |
| 11 | Pd(OAc)2 | K2CO3 | DCE | 45 |
| 12 | Pd(OAc)2 | Cs2CO3 | DCE | Trace |
| 13 | Pd(OAc)2 | HOAc | DCE | 18 |
| 14 | Pd(OAc)2 | CF3COOH | DCE | N.D. |
| 15 | PdCl2 | PhCOOK | DCE | 83 |
| 16 | Pd(PPh3)4 | PhCOOK | DCE | 36 |
| 17 | Pd2(dba)3 | PhCOOK | DCE | 30 |
| 18 | Pd(CH3CN)2Cl2 | PhCOOK | DCE | 80 |
| Entry | Catalyst/mol% | Additive | Solvent | Yieldb/% |
|---|---|---|---|---|
| 1 | Pd(OAc)2 | KOAc | 1,4-Dioxane | 61 |
| 2 | Pd(OAc)2 | PhCOOK | 1,4-Dioxane | 68 |
| 3 | Pd(OAc)2 | PhCOOK | DMF | N.D. |
| 4 | Pd(OAc)2 | PhCOOK | Xylene | 82 |
| 5 | Pd(OAc)2 | PhCOOK | DMSO | N.D. |
| 6 | Pd(OAc)2 | PhCOOK | t-BuOH | 75 |
| 7 | Pd(OAc)2 | PhCOOK | DCE | 86 |
| 8 | Pd(OAc)2 | — | DCE | Trace |
| 9 | Pd(OAc)2 | AgOAc | DCE | Trace |
| 10 | Pd(OAc)2 | NaOAc | DCE | 49 |
| 11 | Pd(OAc)2 | K2CO3 | DCE | 45 |
| 12 | Pd(OAc)2 | Cs2CO3 | DCE | Trace |
| 13 | Pd(OAc)2 | HOAc | DCE | 18 |
| 14 | Pd(OAc)2 | CF3COOH | DCE | N.D. |
| 15 | PdCl2 | PhCOOK | DCE | 83 |
| 16 | Pd(PPh3)4 | PhCOOK | DCE | 36 |
| 17 | Pd2(dba)3 | PhCOOK | DCE | 30 |
| 18 | Pd(CH3CN)2Cl2 | PhCOOK | DCE | 80 |
| Entry | α-Bromo ketones | Product | Yieldb/% | Entry | α-Bromo ketones | Product | Yieldb/% |
|---|---|---|---|---|---|---|---|
| 1 | | 3f | 82 (74c) | 6 | | 3k | 70 |
| 2 | | 3g | 79 | 7 | | 3l | 78 |
| 3 | | 3h | 64 | 8 | | 3m | 71 |
| 4 | | 3i | 55 | 9 | | 3n | 52 |
| 5 | | 3j | 76 | 10d | | 3o | 56 |
| Entry | α-Bromo ketones | Product | Yieldb/% | Entry | α-Bromo ketones | Product | Yieldb/% |
|---|---|---|---|---|---|---|---|
| 1 | | 3f | 82 (74c) | 6 | | 3k | 70 |
| 2 | | 3g | 79 | 7 | | 3l | 78 |
| 3 | | 3h | 64 | 8 | | 3m | 71 |
| 4 | | 3i | 55 | 9 | | 3n | 52 |
| 5 | | 3j | 76 | 10d | | 3o | 56 |
| [1] |
For representative reviews on C—H functionalization, see: (a) Alberico, D.; Scott, M. E.; Lautens, M.; Chem. Rev. 2007, 107,174.
pmid: 31904219 |
|
(b) Ackermann, L. Chem. Commun. 2010, 46,4866.
pmid: 31904219 |
|
|
(c) Li, H.; Li, B.-J.; Shi, Z.-J. Catal. Sci. Technol. 2011, 1,191.
pmid: 31904219 |
|
|
(d) Rouquet, G.; Chatani, N. Angew. Chem. Int. Ed. 2013, 52,11726.
pmid: 31904219 |
|
|
(e) Daugulis, O.; Roane, J.; Tran, L.D. Acc. Chem. Res. 2015, 48,1053.
doi: 10.1021/ar5004626 pmid: 31904219 |
|
|
(f) Chen, Z.; Wang, B.; Zhang, J.; Yu, W.; Liu, Z.; Zhang, Y. Org. Chem. Front. 2015, 2,1107.
pmid: 31904219 |
|
|
(g) He, G.; Wang, B.; Nack, W.A.; Chen, G. Acc. Chem. Res. 2016, 49,635.
doi: 10.1021/acs.accounts.6b00022 pmid: 31904219 |
|
|
(h) Dong, Z.; Ren, Z.; Thompson, S.J.; Xu, Y.; Dong, G. Chem. Rev. 2017, 117,9333.
pmid: 31904219 |
|
|
(i) Saint-Denis, T.G.; Zhu, R.-Y.; Chen, G.; Wu, Q.-F.; Yu, J.-Q. Science 2018, 359,eaao4798.
doi: 10.1126/science.aao4798 pmid: 31904219 |
|
|
(j) Sambiagio, C.; Schönbauer, D.; Blieck, R.; Dao-Huy, T.; Pototschnig, G.; Schaaf, P.; Wiesinger, T.; Zia, M.F.; Wencel-Delord, J.; Besset, T.; Maes, B.U. W.; Schnürch, M. Chem. Soc. Rev. 2018, 47,6603.
pmid: 31904219 |
|
|
(k) Rej, S.; Ano, Y.; Chatani, N. Chem. Rev. 2020, 120,1788.
doi: 10.1021/acs.chemrev.9b00495 pmid: 31904219 |
|
|
(l) Liao, G.; Wu, Y.-J.; Shi, B.-F. Acta Chim. Sinica 2020, 78,289. (in Chinese)
pmid: 31904219 |
|
|
( 廖港, 吴勇杰, 史炳锋, 化学学报, 2020, 78,289.)
pmid: 31904219 |
|
|
(m) Liu, Y.-H.; Xia, Y.-N.; Shi, B.-F. Chin. J. Chem. 2020, 38,635.
pmid: 31904219 |
|
| [2] |
For representative reviews on C—H functionalization logic in natural products and medicinal compounds, see: (a) Godula, K.; Sames, D.; Science 2006, 312,67.
pmid: 26507237 |
|
(b) Gutekunst, W.R.; Baran, P.S. Chem. Soc. Rev. 2011, 40,1976.
pmid: 26507237 |
|
|
(c) Yamaguchi, J.; Yamaguchi, A.D.; Itami, K. Angew. Chem. Int. Ed. 2012, 51,8960.
pmid: 26507237 |
|
|
(d) Chen, D.Y. K.; Youn, S.W. Chem.-Eur. J. 2012, 18,9452.
pmid: 26507237 |
|
|
(e) Cernak, T.; Dykstra, K.D.; Tyagarajan, S.; Vachal, P.; Krska, S.W. Chem. Soc. Rev. 2016, 45,546.
doi: 10.1039/c5cs00628g pmid: 26507237 |
|
|
(f) Wang, W.; Lorion, M.M.; Shah, J.; Kapdi, A.R.; Ackermann, L. Angew. Chem. Int. Ed. 2018, 57,14700.
pmid: 26507237 |
|
| [3] |
(a) Huang, L.; Li, Q.; Wang, C.; Qi, C. J. Org. Chem. 2013, 78,3030.
doi: 10.1021/jo400017v pmid: 25000283 |
|
(b) Huang, L.; Sun, X.; Li, Q.; Qi, C. J. Org. Chem. 2014, 79,6720.
pmid: 25000283 |
|
| [4] |
(a) Nadres, E.T.; Santos, G.I. F.; Shabashov, D.; Daugulis, O. J. Org. Chem. 2013, 78,9689.
pmid: 25399697 |
|
(b) Yu, X.; Yang, F.; Wu, Y.; Wu, Y. Org. Lett. 2019, 21,1726.
pmid: 25399697 |
|
|
(c) Shi, Y.; Yang, F.; Wu, Y. Org. Biomol. Chem. 2020, 18,4628.
pmid: 25399697 |
|
|
(d) Li, Z.; Sun, S.; Qiao, H.; Yang, F.; Zhu, Y.; Kang, J.; Wu, Y.; Wu, Y. Org. Lett. 2016, 18,4594.
pmid: 25399697 |
|
|
(e) Guan, D.; Han, L.; Wang, L.; Song, H.; Chu, W.; Sun, Z. Chem. Lett. 2015, 44,743.
pmid: 25399697 |
|
|
(f) Wang, L.; Yang, M.; Liu, X.; Song, H.; Han, L.; Chu, W.; Sun, Z. Appl. Organomet. Chem. 2016, 30,680.
pmid: 25399697 |
|
|
(g) Iwasaki, M.; Kaneshika, W.; Tsuchiya, Y.; Nakajima, K.; Nishihara, Y. J. Org. Chem. 2014, 79,11330.
doi: 10.1021/jo502274t pmid: 25399697 |
|
| [5] |
For representative reviews on the biological activities of aporphine alkaloids,see: (a) Guinaudeau, H.; Leboeuf, M.; Cavé, A. J. Nat. Prod. 1994, 57,1033.
pmid: 14700200 |
|
(b) Ríos, J.L.; Máñez, S.; Giner, R.M.; Recio, M.C. In The Alkaloids: Chemistry and Biology,Ed.: Cordell, G. A., Academic Press, 1999, Vol.53, p.57.
pmid: 14700200 |
|
|
For representative reviews on the biological activities of aristolactam alkaloids,see: (c) Kumar, V.; Poonam; Prasad, A.K.; Parmar, V.S. Nat. Prod. Rep. 2003, 20,565.
doi: 10.1039/b303648k pmid: 14700200 |
|
|
(d) Bentley, K.W. Nat. Prod. Rep. 2006, 23,444.
pmid: 14700200 |
|
| [6] |
For representative examples on the biological activities of aporphine alkaloids,see: (a) Zhang, A.; Zhang, Y.; Branfman, A. R.; Baldessarini, R. J.; Neumeyer, J. L. J. Med. Chem. 2007, 50,171.
pmid: 9584402 |
|
(b) Stevigny, C.; Bailly, C.; Quetin-Leclercq, J. Anti-Cancer Agents Med. Chem. 2005, 5,173.
pmid: 9584402 |
|
|
(c) Mohamed, S.M.; Hassan, E.M.; Ibrahim, N.A. Nat. Prod. Res. 2009, 24,1395.
pmid: 9584402 |
|
|
(d) Boustie, J.; Stigliani, J.-L.; Montanha, J.; Amoros, M.; Payard, M.; Girre, L. J. Nat. Prod. 1998, 61,480.
doi: 10.1021/np970382v pmid: 9584402 |
|
| [7] |
For representative examples on the biological activities of aristolactam alkaloids, see: (a) Chia, Y.-C.; Chang, F.-R.; Teng, C.-M.; Wu, Y.-C. J. Nat. Prod. 2000, 63,1160.
doi: 10.1021/np000063v pmid: 1431939 |
|
(b) Zhang, Y.-N.; Zhong, X.-G.; Zheng, Z.-P.; Hu, X.-D.; Zuo, J.-P.; Hu, L.-H. Bioorg. Med. Chem. 2007, 15,988.
pmid: 1431939 |
|
|
(c) Lee, H.S.; Han, D.S. J. Nat. Prod. 1992, 55,1165.
pmid: 1431939 |
|
| [8] |
Song, J.; Chen, W.; Zhao, Y.; Li, C.; Liang, G.; Huang, L. RSC Adv. 2016, 6,54984.
|
| [9] |
(a) De Kimpe, N.; Verhé, R. α-Haloketones, α-Haloaldehydes and α-Haloimines, Wiley, New York, 1988, p. 1.
pmid: 26700265 |
|
(b) Yasuda, M.; Tsuji, S.; Shigeyoshi, Y.; Baba, A. J. Am. Chem. Soc. 2002, 124,7440.
pmid: 26700265 |
|
|
(c) Malosh, C.F.; Ready, J.M. J. Am. Chem. Soc. 2004, 126,10240.
pmid: 26700265 |
|
|
(d) Liu, C.; He, C.; Shi, W.; Chen, M.; Lei, A. Org. Lett. 2007, 9,5601.
pmid: 26700265 |
|
|
(e) Lundin, P.M.; Fu, G.C. J. Am. Chem. Soc. 2010, 132,11027.
doi: 10.1021/ja105148g pmid: 26700265 |
|
|
(f) Huang, K.; Li, G.; Huang, W.-P.; Yu, D.-G.; Shi, Z.-J. Chem. Commun. 2011, 47,7224.
pmid: 26700265 |
|
|
(g) Mao, J.; Liu, F.; Wang, M.; Wu, L.; Zheng, B.; Liu, S.; Zhong, J.; Bian, Q.; Walsh, P.J. J. Am. Chem. Soc. 2014, 136,17662.
pmid: 26700265 |
|
|
(h) Shu, W.-M.; Ma, J.-R.; Zheng, K.-L.; Wu, A.-X. Org. Lett. 2016, 18,196.
doi: 10.1021/acs.orglett.5b03236 pmid: 26700265 |
|
|
(i) Liu, W.; Cao, W.; Hu, H.; Lin, L.; Feng, X. Chem. Commun. 2018, 54,8901.
pmid: 26700265 |
|
| [10] |
For representative examples on the α-halo esters and ketones can be employed as the alkylation reagent in C—H functionalization, see: (a) Hennessy, E. J.; Buchwald, S. L. J. Am. Chem. Soc. 2003, 125,12084.
doi: 10.1021/ja037546g pmid: 30457342 |
|
(b) Liu, C.; Liu, D.; Zhang, W.; Zhou, L.; Lei, A. Org. Lett. 2013, 15,6166.
doi: 10.1021/ol403021p pmid: 30457342 |
|
|
(c) Nakatani, A.; Hirano, K.; Satoh, T.; Miura, M. Chem.-Eur. J. 2013, 19,7691.
pmid: 30457342 |
|
|
(d) Yu, D.-G.; de Azambuja, F.; Glorius, F. Angew. Chem. Int. Ed. 2014, 53,2754.
pmid: 30457342 |
|
|
(e) Xie, C.; Dai, Z.; Niu, Y.; Ma, C. J. Org. Chem. 2018, 83,2317.
pmid: 30457342 |
|
|
(f) Li, J.; Zhang, Z.; Tang, M.; Zhang, X.; Jin, J. Org. Lett. 2016, 18,3898.
pmid: 30457342 |
|
|
(g) Zhou, J.; Li, J.; Li, Y.; Wu, C.; He, G.; Yang, Q.; Zhou, Y.; Liu, H. Org. Lett. 2018, 20,7645.
pmid: 30457342 |
|
| [11] |
During preparation of the manuscript, similar reaction was reported by the Wu group: Wang, X.; Feng, C.; Yang, F.; Wu, Y. Org. Biomol. Chem. 2019, 17,4865.
pmid: 31041981 |
| [12] |
He, G.; Lu, C.; Zhao, Y.; Nack, W.A.; Chen, G. Org. Lett. 2012, 14,2944.
pmid: 22670815 |
| [13] |
Hase, T. Synthesis 1980,36.
|
| [14] |
(a) Zaitsev, V.G.; Shabashov, D.; Daugulis, O. J. Am. Chem. Soc. 2005, 127,13154.
doi: 10.1021/ja054549f pmid: 20175511 |
|
(b) Shabashov, D.; Daugulis, O. J. Am. Chem. Soc. 2010, 132,3965.
pmid: 20175511 |
|
|
(c) Nadres, E.T.; Daugulis, O. J. Am. Chem. Soc. 2011, 134,7.
pmid: 20175511 |
|
|
(d) Tran, L.D.; Daugulis, O. Angew. Chem., nt. Ed. 2012, 51,5188.
pmid: 20175511 |
|
|
(e) He, G.; Chen, G. Angew. Chem., nt. Ed. 2011, 50,5192.
pmid: 20175511 |
|
| [15] |
(a) Xie, Y.; Yang, Y.; Huang, L.; Zhang, X.; Zhang, Y. Org. Lett. 2012, 14,1238.
doi: 10.1021/ol300037p pmid: 22356490 |
|
(b) Zhang, M.; Li, R.; Yang, Z.; Feng, R. Chin. J. Org. Chem. 2020, 40,714. (in Chinese)
pmid: 22356490 |
|
|
( 张梦帆, 李瑞鹏, 杨震, 冯若昆, 有机化学, 2020, 40,714.)
pmid: 22356490 |
|
| [16] |
(a) Xie, A.; Cao, M.; Liu, Y.; Feng, L.; Hu, X.; Dong, W. Eur. J. Org. Chem. 2014,436.
pmid: 24588126 |
|
(b) Li, Q.; Zhang, S.-Y.; He, G.; Ai, Z.; Nack, W.A.; Chen, G. Org. Lett. 2014, 16,1764.
pmid: 24588126 |
|
| [17] |
DeRuiter, J.; Swearingen, B.E.; Wandrekar, V.; Mayfield, C.A. J. Med. Chem. 1989, 32,1033.
pmid: 2496229 |
| [18] |
Jia, X.; Huang, Q.; Li, J.; Li, S.; Yang, Q. Synlett 2007,0806.
|
| [19] |
Yang, N.C.; Lenz, G.R.; Shani, A. Tetrahedron Lett. 1966, 7,2941.
|
| [20] |
Ying, J.; Fu, L.-Y.; Zhong, G.; Wu, X.-F. Org. Lett. 2019, 21,5694.
pmid: 31246481 |
| [1] | 孟宪强, 杨艺, 梁万洁, 王靖涛, 张荣葵, 刘会. 钯催化联烯胺区域选择性芳基酚氧化反应[J]. 有机化学, 2024, 44(1): 224-231. |
| [2] | 王兢睿, 冯永奎, 王能中, 黄年玉, 姚辉. 钯催化立体选择性合成硝基烷类β-碳糖苷[J]. 有机化学, 2023, 43(9): 3216-3225. |
| [3] | 程春霞, 吴露平, 沙风, 伍新燕. 手性叔膦-酰胺不对称催化香豆素与Morita-Baylis-Hillman碳酸酯之间的插烯烯丙基烷基化反应[J]. 有机化学, 2023, 43(9): 3188-3195. |
| [4] | 鄢伯钰, 吴阶良, 邓金飞, 陈丹, 叶秀深, 姚秋丽. 光诱导醇的直接脱羟基衍生化研究进展[J]. 有机化学, 2023, 43(9): 3055-3066. |
| [5] | 宋晓, 卿晶, 黎君, 贾雪雷, 吴福松, 黄均荣, 金剑, 游恒志. 铜催化格氏试剂的不对称烯丙基烷基化连续流反应[J]. 有机化学, 2023, 43(9): 3174-3179. |
| [6] | 王玉超, 刘晋彪, 何智涛. 钯催化共轭二烯的不对称氢官能团化[J]. 有机化学, 2023, 43(8): 2614-2627. |
| [7] | 冯莹珂, 王贺, 崔梦行, 孙然, 王欣, 陈阳, 李蕾. 可见光诱导的新型官能化芳基异腈化合物的二氟烷基化环化反应[J]. 有机化学, 2023, 43(8): 2913-2925. |
| [8] | 黄芬, 罗维纬, 周俊. 基于C—H键断裂的多氯烷基化反应研究进展[J]. 有机化学, 2023, 43(7): 2368-2390. |
| [9] | 孙丽, 宋国欣, 韩家乐, 李继玉, 赵月, 杨璐华, 张峰, 赵坤, 毛比明. Morita-Baylis-Hillman加合物和N-羟基邻苯二甲酰亚胺的电化学烯丙基烷基化形成C(sp3)—C(sp3)键[J]. 有机化学, 2023, 43(4): 1574-1583. |
| [10] | 赵金晓, 魏彤辉, 柯森, 李毅. 可见光催化合成二氟烷基取代的多环吲哚化合物[J]. 有机化学, 2023, 43(3): 1102-1114. |
| [11] | 向勋, 何照林, 董秀琴. 钯和手性磷酸协同催化高效构建手性分子的研究进展[J]. 有机化学, 2023, 43(3): 791-808. |
| [12] | 周鹏, 朱伟明, 张建涛, 肖朵朵, 郭祥峰, 刘卫兵. 钴催化芳基烯烃氧烷基化反应: 快速获得α-烷基取代苯乙酮衍生物[J]. 有机化学, 2023, 43(11): 3939-3944. |
| [13] | 孙美娇, 谭晶, 谭玉, 彭进松, 陈春霞. 钯催化3-(2-氨基嘧啶-4-基)吲哚2位C—H键芳基化反应的研究[J]. 有机化学, 2023, 43(11): 3945-3959. |
| [14] | 刘甜甜, 段新红. 不对称傅-克反应在构建手性3-取代吲哚中的研究进展[J]. 有机化学, 2023, 43(11): 3695-3712. |
| [15] | 涂志, 余金生, 周剑. 溴二氟甲基三甲基硅烷的合成及其在有机合成中的应用[J]. 有机化学, 2023, 43(10): 3491-3507. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||