有机化学 ›› 2023, Vol. 43 ›› Issue (10): 3491-3507.DOI: 10.6023/cjoc202306024 上一篇 下一篇
所属专题: 有机氟化学虚拟合辑; 有机硅化学专辑-2023
综述与进展
收稿日期:2023-06-27
修回日期:2023-08-23
发布日期:2023-08-30
基金资助:
Zhi Tua, Jinsheng Yua( ), Jian Zhoua,b,c
), Jian Zhoua,b,c
Received:2023-06-27
Revised:2023-08-23
Published:2023-08-30
Contact:
*E-mail: Supported by:文章分享

溴二氟甲基三甲基硅烷(TMSCF2Br)自2011年首次被作为二氟卡宾前体报道以来, 已发展成为一种重要的二氟甲(烷)基化试剂, 并在有机合成中得到了广泛应用. 在简要介绍TMSCF2Br合成方法的基础上, 重点阐述了利用TMSCF2Br作为二氟卡宾前体或三甲基硅基二氟甲基自由基前体实现不同底物的系列二氟甲基或氟烷基化反应的研究进展. 藉此对TMSCF2Br在有机合成反应的活化方式和机理及其应用优势与不足等进行分析讨论, 为从事有机合成和有机氟化学相关的科研人员提供一些参考与启发.
涂志, 余金生, 周剑. 溴二氟甲基三甲基硅烷的合成及其在有机合成中的应用[J]. 有机化学, 2023, 43(10): 3491-3507.
Zhi Tu, Jinsheng Yu, Jian Zhou. Synthesis of (Bromodifluoromethyl)trimethylsilane and Its Applications in Organic Synthesis[J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3491-3507.
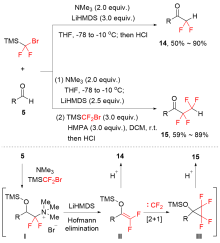
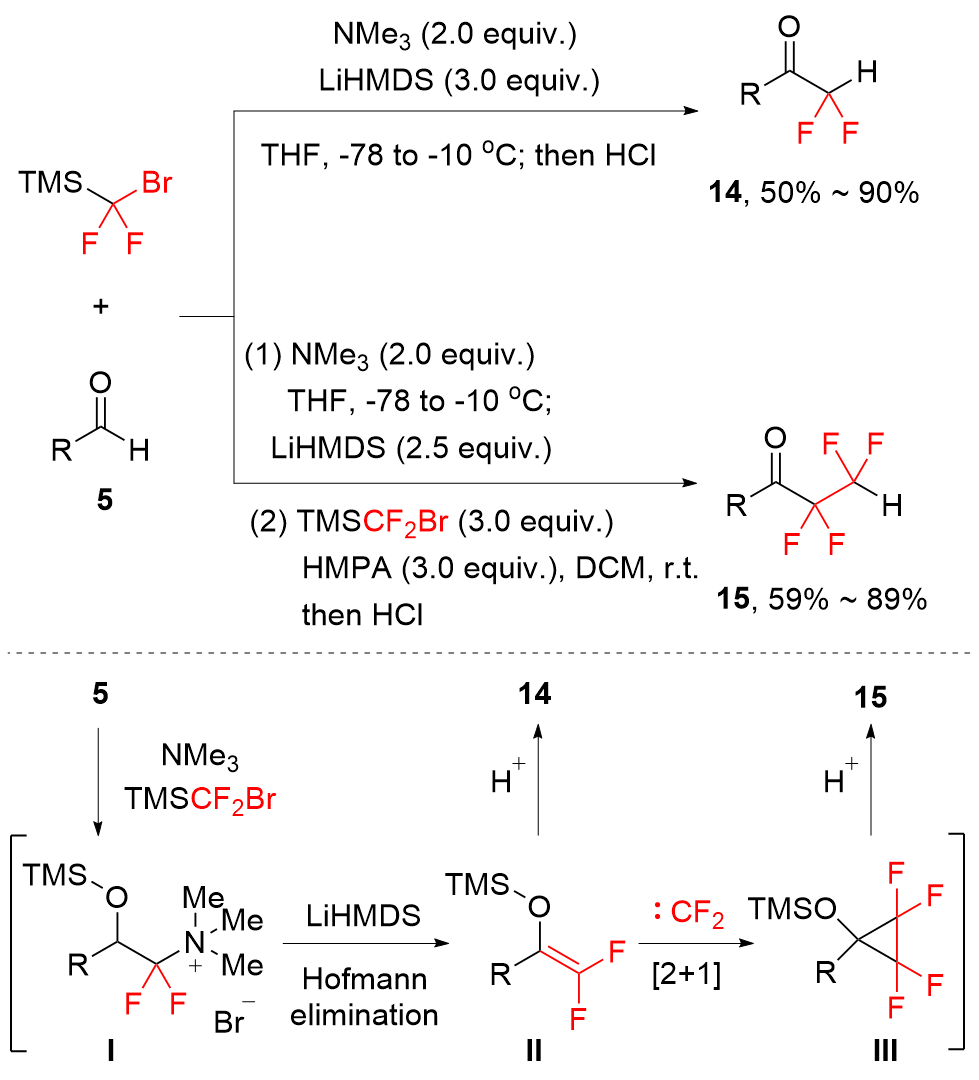
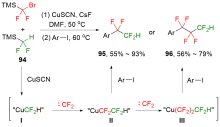
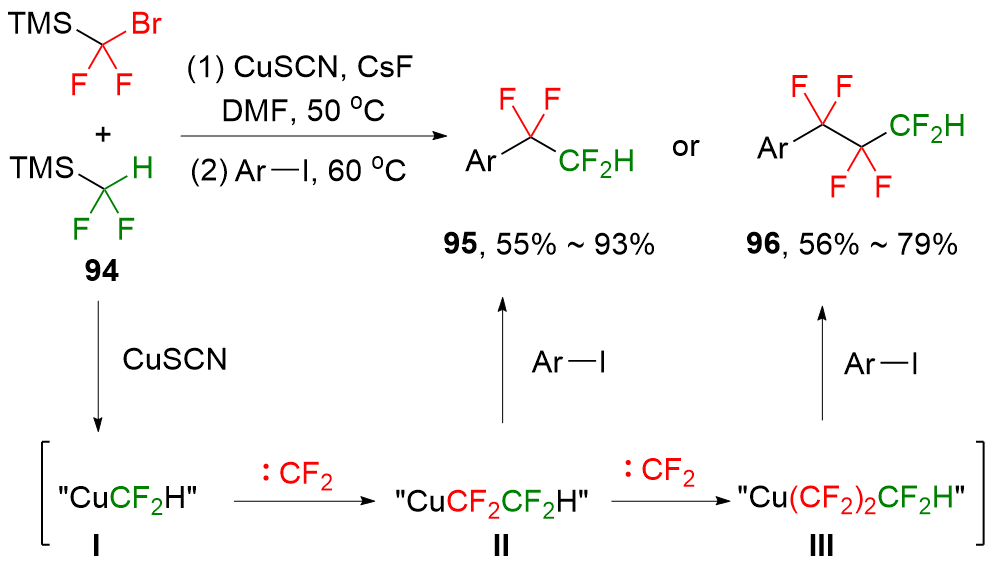
| [1] |
(a) Uneyama K. Organofluorine Chemistry, Blackwell, Oxford, 2006.
|
|
(b) Chambers R. D. Fluorine in Organic Chemistry, Blackwell, Oxford, 2004.
|
|
|
(c) Kirsch P. Modern Fluoroorganic Chemistry: Synthesis Reactivity, Applications, 2nd ed., Wiley-VCH, Weinheim, 2013.
|
|
| [2] |
(a) Erickson J. A.; McLoughlin J. I. J. Org. Chem. 1995, 60, 1626.
doi: 10.1021/jo00111a021 pmid: 32027173 |
|
(b) Sap J. B.; Meyer C. F.; Straathof N. J.; Iwumene N.; Am Ende C. W.; Trabanco A. A.; Gouverneur V. J. Am. Chem. Soc. 2017, 139, 9325.
doi: 10.1021/jacs.7b04457 pmid: 32027173 |
|
|
(c) Zafrani Y.; Saphier S.; Gershonov E. Future Med. Chem. 2020, 12, 361.
doi: 10.4155/fmc-2019-0309 pmid: 32027173 |
|
|
(d) Sap J. B.; Meyer C. F; Straathof N. J.; Iwumene N.; Am Ende C. W.; Trabanco A. A.; Gouverneur V. Chem. Soc. Rev. 2021, 50, 8214.
doi: 10.1039/D1CS00360G pmid: 32027173 |
|
| [3] |
(a) Prakash G. K. S.; Yudin A. K. Chem. Rev. 1997, 97, 757.
pmid: 11848888 |
|
(b) Liu X.; Xu C.; Wang M.; Liu Q. Chem. Rev. 2015, 115, 683.
doi: 10.1021/cr400473a pmid: 11848888 |
|
|
(c) Dilman A. D.; Levin V. V. Mendeleev Commun. 2015, 25, 239.
doi: 10.1016/j.mencom.2015.07.001 pmid: 11848888 |
|
|
(d) Rong J.; Ni C.-F.; Hu J.-B. Asian J. Org. Chem. 2017, 6, 139.
doi: 10.1002/ajoc.v6.2 pmid: 11848888 |
|
|
(e) Chen D.-B.; Gao X.; Song S.-R.; Kou M.; Ni C.-F.; Hu J.-B. Sci. Sin.: Chim. 2023, 53, 375.
pmid: 11848888 |
|
| [4] |
Wang F.; Zhang W.; Zhu J.-M.; Li H.-F.; Huang K.-W.; Hu J.-B. Chem. Commun. 2011, 47, 2411
doi: 10.1039/C0CC04548A |
| [5] |
Li L.-C.; Wang F.; Ni C.-F.; Hu J.-B. Angew. Chem., Int. Ed. 2013, 52, 12390.
doi: 10.1002/anie.v52.47 |
| [6] |
(a) Hu J.-B.; Zhang W.; Wang F. Chem. Commun. 2009, 7465.
pmid: 28051859 |
|
(b) Hu J.-B. J. Fluorine Chem. 2009, 130, 1130.
doi: 10.1016/j.jfluchem.2009.05.016 pmid: 28051859 |
|
|
(c) Qing F.-L.; Zheng F. Synlett 2011, 2011, 1052.
doi: 10.1055/s-0030-1259947 pmid: 28051859 |
|
|
(d) Meanwell N. A. J. Med. Chem. 2011, 54, 2529.
doi: 10.1021/jm1013693 pmid: 28051859 |
|
|
(e) Ni C.-F.; Hu J.-B. Synthesis 2014, 46, 842.
doi: 10.1055/s-00000084 pmid: 28051859 |
|
|
(f) Belhomme M. C.; Besset T.; Poisson T.; Pannecoucke X. Chem.-Eur. J. 2015, 21, 12836.
doi: 10.1002/chem.v21.37 pmid: 28051859 |
|
|
(g) Zafrani Y.; Yeffet D.; Sod-Moriah G.; Berliner A.; Amir D.; Marciano D.; Gershonov E.; Saphier S. J. Med. Chem. 2017, 60, 797.
doi: 10.1021/acs.jmedchem.6b01691 pmid: 28051859 |
|
| [7] |
(a) Wang J.; Sánchez-Roselló M.; Aceña J. L.; Del Pozo C.; Srochinsky A. E.; Fustero S.; Soloshonok V. A.; Liu H. Chem. Rev. 2014, 114, 2432.
doi: 10.1021/cr4002879 pmid: 19296694 |
|
(b) Chowdhury M. A.; Abdellatif K. R.; Dong Y.; Das D.; Suresh M. R.; Knaus E. E. J. Med. Chem. 2009, 52, 1525.
doi: 10.1021/jm8015188 pmid: 19296694 |
|
|
(c) Gewehr M.; Gladwin R. J.; Brahm L. US 2012/0245031, 2012.
pmid: 19296694 |
|
|
(d) Pérez R. A.; Sánchez-Brunete C.; Miguel E.; Tadeo J. L. J. Agric. Food Chem. 1998, 46, 1864.
doi: 10.1021/jf970854b pmid: 19296694 |
|
| [8] |
(a) Wang X.; Pan S.-T.; Luo Q.-Y.; Wang Q.; Ni C.-F.; Hu J.-B. J. Am. Chem. Soc. 2022, 144, 12202.
doi: 10.1021/jacs.2c03104 pmid: 35786906 |
|
(b) Trang B.; Li Y.-L.; Xue X.-S.; Ateia M.; Houk K. N.; Dichtel W. R. Science 2022, 377, 839.
doi: 10.1126/science.abm8868 pmid: 35786906 |
|
|
(c) Tsyrulnikova A. S.; Vershilov S. V.; Popova L. M.; Lebedev N. V.; Litvinenko E. V.; Ismagilov N. G. J. Fluorine Chem. 2022, 257, 109972.
pmid: 35786906 |
|
| [9] |
Evich M. G.; Davis M. J. B.; McCord J. P.; Acrey B.; Awkerman J. A.; Knappe D. R. U.; Lindstrom A. B.; Speth T. F.; Tebes-Stevens C.; Strynar M. J.; Wang Z.-Y.; Weber E. J.; Henderson W. M.; Washington J. W. Science 2022, 375, eabg9065.
|
| [10] |
(a) Krishnamoorthy S.; Prakash G. K. S. Synthesis 2017, 49, 3394.
doi: 10.1055/s-0036-1588489 |
|
(b) Yerien D. E.; Barata-Vallejo S.; Postigo A. Chem. Eur. J. 2017, 23, 14676.
doi: 10.1002/chem.v23.59 |
|
|
(c) Sap J. B.; Meyer C. F.; Straathof N. J.; Iwumene N.; Am Ende C. W.; Trabanco A. A.; Gouverneur V. Chem. Soc. Rev. 2021, 50, 8214.
doi: 10.1039/D1CS00360G |
|
|
(d) Dilman A. D.; Levin V. V. Acc. Chem. Res. 2018, 51, 1272.
doi: 10.1021/acs.accounts.8b00079 |
|
| [11] |
(a) Broicher V.; Geffken D. J. Organomet. Chem. 1990, 381, 315.
doi: 10.1016/0022-328X(90)80061-4 |
|
(b) Ruppert I.; Schlich K.; Volbach W. Tetrahedron Lett. 1984, 25, 2195.
doi: 10.1016/S0040-4039(01)80208-2 |
|
|
(c) Yudin A. K.; Prakash G. K. S.; Deffieux D.; Bradley M.; Bau R.; Olah G. A. J. Am. Chem. Soc. 1997, 119, 1572.
doi: 10.1021/ja962990n |
|
| [12] |
Kosobokov M. D.; Dilman A. D.; Levin V. V.; Struchkova M. I. J. Org. Chem. 2012, 77, 5850.
doi: 10.1021/jo301094b pmid: 22708637 |
| [13] |
(a) Kosobokov M. D.; Levin V. V.; Struchkova M. I.; Dilman A. D. Org. Lett. 2014, 16, 3784.
doi: 10.1021/ol501674n pmid: 24968144 |
|
(b) Levin V. V.; Smirnov V. O.; Struchkova M. I.; Dilman A. D. J. Org. Chem. 2015, 80, 9349.
doi: 10.1021/acs.joc.5b01590 pmid: 24968144 |
|
| [14] |
Maslov A. S.; Smirnov V. O.; Struchkova M. I.; Arkhipov D. E.; Dilman A. D. Tetrahedron Lett. 2015, 56, 5048.
doi: 10.1016/j.tetlet.2015.07.018 |
| [15] |
Xie Q.-Q.; Zhu Z.-Y.; Ni C.-F.; Hu J.-B. Org. Lett. 2019, 21, 9138.
doi: 10.1021/acs.orglett.9b03520 |
| [16] |
Liu A.; Ni C.-F.; Xie Q.-Q.; Hu J.-B. Angew. Chem., Int. Ed. 2022, 61, e202115467.
|
| [17] |
Liu A.; Ni C.-F.; Xie Q.-Q.; Hu J.-B. Angew. Chem., Int. Ed. 2023, 62, e202217088.
|
| [18] |
Tsymbal A. V.; Kosobokov M. D.; Levin V. V.; Struchkova M. I.; Dilman A. D. J. Org. Chem. 2014, 79, 7831.
doi: 10.1021/jo501644m pmid: 25116859 |
| [19] |
Trifonov A. L.; Zemtsov A. A.; Levin V. V.; Struchkova M. I.; Dilman A. D. Org. Lett. 2016, 18, 3458.
doi: 10.1021/acs.orglett.6b01641 pmid: 27336618 |
| [20] |
Trifonov A. L.; Dilman A. D. Org. Lett. 2021, 23, 6977
doi: 10.1021/acs.orglett.1c02603 |
| [21] |
Trifonov A. L.; Levin V. V.; Struchkova M. I.; Dilman A. D. Org. Lett. 2017, 19, 5304.
doi: 10.1021/acs.orglett.7b02601 pmid: 28915059 |
| [22] |
(a) Glenadel Q.; Ismalaj E.; Billard T. J. Org. Chem. 2016, 81, 8268.
doi: 10.1021/acs.joc.6b01344 pmid: 34823360 |
|
(b) Fang Y.; Li X.; Liu C.-Y.; Tang J.; Chen Z.-P. J. Org. Chem. 2021, 86, 18081.
doi: 10.1021/acs.joc.1c02349 pmid: 34823360 |
|
| [23] |
(a) Kosobokov M. D.; Levin V. V.; Struchkova M. I.; Dilman A. D. Org. Lett. 2015, 17, 760.
doi: 10.1021/acs.orglett.5b00097 pmid: 25965426 |
|
(b) Fedorov O. V.; Kosobokov M. D.; Levin V. V.; Struchkova M. I.; Dilman A. D. J. Org. Chem. 2015, 80, 5870.
doi: 10.1021/acs.joc.5b00904 pmid: 25965426 |
|
| [24] |
(a) Song X.-N.; Chang J.; Zhu D.-S.; Li J.-H.; Xu C.; Liu Q.; Wang M. Org. Lett. 2015, 17, 1712.
doi: 10.1021/acs.orglett.5b00488 |
|
(b) Chang J.; Song X.-N.; Huang W.-Q.; Zhu D.-S.; Wang M. Chem. Commun. 2015, 51, 15362.
doi: 10.1039/C5CC06825H |
|
| [25] |
(a) Song X.-N.; Tian S.-Q.; Zhao Z.-M.; Zhu D.-S.; Wang M. Org. Lett. 2016, 18, 3414.
doi: 10.1021/acs.orglett.6b01567 pmid: 28332846 |
|
(b) Chang J.; Xu C.; Gao J.; Gao F.-Y.; Zhu D.-S.; Wang M. Org. Lett. 2017, 19, 1850.
doi: 10.1021/acs.orglett.7b00611 pmid: 28332846 |
|
| [26] |
Zhu J.; Xu M.-H.; Gong B.-H.; Lin A.-J.; Gao S. Org. Lett. 2023, 25, 3271.
doi: 10.1021/acs.orglett.3c01007 |
| [27] |
(a) Xie Q.-Q.; Ni C.-F.; Zhang R.-Y.; Li L.-C.; Rong J.; Hu J.-B. Angew. Chem., Int. Ed. 2017, 56, 3206.
doi: 10.1002/anie.v56.12 |
|
(b) Zhang R.-Y.; Ni C.-F.; Xie Q.-Q.; Hu J.-B. Tetrahedron 2020, 76, 131676.
doi: 10.1016/j.tet.2020.131676 |
|
| [28] |
Smirnov V. O.; Maslov A. S.; Struchkova M. I.; Arkhipov D. E.; Dilman A. D. Mendeleev Commun. 2015, 25, 452.
doi: 10.1016/j.mencom.2015.11.018 |
| [29] |
Krishnamurti V.; Barrett C.; Ispizua-Rodriguez X.; Coe M.; Prakash G. K. S. Org. Lett. 2019, 21, 9377.
doi: 10.1021/acs.orglett.9b03604 pmid: 31742416 |
| [30] |
(a) Zhang R.-Y.; Li Q.-G.; Ni C.-F.; Hu J.-B. Chem. Eur. J. 2021, 27, 17773.
doi: 10.1002/chem.v27.71 |
|
(b) Zhu X.-J.; Wei J.; Hu C.-X.; Xiao Q.-T.; Cai L.-T.; Wang H.; Xie Y.-Y.; Sheng R. Eur. J. Org. Chem. 2022, e202200629.
|
|
|
(c) Sheng H.-Y.; Su J.-K.; Li X.; Song Q.-L. CCS Chem. 2022, 4, 3820.
doi: 10.31635/ccschem.022.202101576 |
|
| [31] |
Huang Y.-B.; Lin Z.-M.; Chen Y.; Fang S.-J.; Jiang H.-F.; Wu W.-Q. Org. Chem. Front. 2019, 6, 2462.
doi: 10.1039/C9QO00506D |
| [32] |
Kim Y.; Heo J.; Kim D.; Chang S.; Seo S. Nat. Commun. 2020, 11, 4761.
doi: 10.1038/s41467-020-18557-8 |
| [33] |
Hayashi H.; Takano H.; Katsuyama H.; Harabuchi Y.; Maeda S.; Mita T. Chem. Eur. J. 2021, 27, 10040.
doi: 10.1002/chem.v27.39 |
| [34] |
Zhu Z.; Krishnamurti V.; Ispizua-Rodriguez X.; Barrett C.; Prakash G. K. S. Org. Lett. 2021, 23, 6494.
doi: 10.1021/acs.orglett.1c02305 |
| [35] |
Zhu Z.-Y.; Xu Y.-J.; Krishnamurti V.; Koch C. J.; Ispizua-Rodriguez X.; Barrett C.; Prakash G. K. S. J. Fluorine Chem. 2022, 261, 110023.
|
| [36] |
Song H.-H.; Li W.-H.; Wang X.-Y.; Wang K.-T.; Li J.-W.; Liu S.; Gao P.; Duan X.-H.; Hu J.-B.; Hu M.-Y. CCS Chem. 2023, DOI: 10.31635/ccschem.023.202302980.
|
| [37] |
(a) Levin V. V.; Zemtsov A. A.; Struchkova M. I.; Dilman A. D. Org. Lett. 2013, 15, 917.
doi: 10.1021/ol400122k pmid: 26664635 |
|
(b) Smirnov V. O.; Maslov A. S.; Levin V. V.; Struchkova M. I.; Dilman A. D. Russ. Chem. Bull. 2014, 63, 2564.
doi: 10.1007/s11172-014-0778-1 pmid: 26664635 |
|
|
(c) Zemtsov A. A.; Kondratyev N. S.; Levin V. V.; Struchkova M. I.; Dilman A. D. J. Org. Chem. 2014, 79, 818.
doi: 10.1021/jo4024705 pmid: 26664635 |
|
|
(d) Smirnov V. O.; Struchkova M. I.; Arkhipov D. E.; Korlyukov A. A.; Dilman A. D. J. Org. Chem. 2014, 79, 11819.
doi: 10.1021/jo5023537 pmid: 26664635 |
|
|
(e) Kondratyev N. S.; Levin V. V.; Zemtsov A. A.; Struchkova M. I.; Dilman A. D. J. Fluorine Chem. 2015, 176, 89.
doi: 10.1016/j.jfluchem.2015.06.001 pmid: 26664635 |
|
|
(f) Zemtsov A. A.; Volodin A. D.; Levin V. V.; Struchkova M. I.; Dilman A. D. Beilstein J. Org. Chem. 2015, 11, 2145.
doi: 10.3762/bjoc.11.231 pmid: 26664635 |
|
|
(g) Zemtsov A. A.; Kondratyev N. S.; Levin V. V.; Struchkova M. I.; Dilman A. D. Russ. Chem. Bull. 2016, 65, 2760.
doi: 10.1007/s11172-016-1649-8 pmid: 26664635 |
|
|
(h) Ashirbaev S. S.; Levin V. V.; Struchkova M. I.; Dilman A. D. J. Fluorine Chem. 2016, 191, 143.
doi: 10.1016/j.jfluchem.2016.07.018 pmid: 26664635 |
|
| [38] |
(a) Wang J.-D.; Tokunaga E.; Shibata N. Chem. Commun. 2018, 54, 8881.
doi: 10.1039/C8CC05135F |
|
(b) Wang Y.-K.; Wang S.-F.; Zhang C.-H.; Zhao T.; Hu Y.-Q.; Zhang M.-W.; Fu Y. Synlett 2021, 32, 1123.
doi: 10.1055/a-1507-5878 |
|
|
(c) Wang Y.-K.; Wang S.-F.; Qiu P.-Y.; Fang L.-Z.; Wang K.; Zhang Y.-W.; Zhao C.-H.; Zhao T. Org. Biomol. Chem. 2021, 19, 4788.
doi: 10.1039/D1OB00511A |
|
| [39] |
Xie Q.-Q.; Zhu Z.-Y.; Li L.-C.; Ni C.-F.; Hu J.-B. Angew. Chem., Int. Ed. 2019, 58, 6405.
doi: 10.1002/anie.v58.19 |
| [40] |
(a) Hu M.-Y.; Ni C.-F.; Li L.-C.; Han Y.-X.; Hu J.-B. J. Am. Chem. Soc. 2015, 137, 14496.
doi: 10.1021/jacs.5b09888 |
|
(b) Zhang Z.-K.; Yu W.-Z.; Wu C.-G.; Wang C.-P.; Zhang Y.; Wang J.-B. Angew. Chem., Int. Ed. 2016, 55, 273.
doi: 10.1002/anie.v55.1 |
|
| [41] |
(a) Fu X.-P.; Xue X.-S.; Zhang X.-Y.; Xiao Y.-L.; Zhang S.; Guo Y.-L.; Leng X.-B.; Houk K. N.; Zhang X.-G. Nat. Chem. 2019, 11, 948.
doi: 10.1038/s41557-019-0331-9 pmid: 31548670 |
|
(b) Zeng X.; Li Y.; Min Q.-Q.; Xue X.-S.; Zhang X.-G. Nat. Chem. 2023, 15, 1064.
doi: 10.1038/s41557-023-01236-8 pmid: 31548670 |
|
| [42] |
Yang R.-Y.; Wang H.; Xu B. Chem. Commun. 2021, 57, 4831.
doi: 10.1039/D1CC01132D |
| [43] |
(a) Zhang R.; Zhang Z.-K.; Zhou Q.; Yu L.-F.; Wang J.-B. Angew. Chem., Int. Ed. 2019, 58, 5744.
doi: 10.1002/anie.v58.17 pmid: 32633508 |
|
(b) Zhang R.; Zhang Z.-K.; Wang K.; Wang J.-B. J. Org. Chem. 2020, 85, 9791.
doi: 10.1021/acs.joc.0c01120 pmid: 32633508 |
|
| [44] |
Wang X.; Pan S.-T.; Luo Q.-Y.; Wang Q.; Ni C.-F.; Hu J.-B. J. Am. Chem. Soc. 2022, 144, 12202.
doi: 10.1021/jacs.2c03104 pmid: 35786906 |
| [45] |
Supranovich V. I.; Levin V. V.; Struchkova M. I.; Korlyukov A. A.; Dilman A. D. Org. Lett. 2017, 19, 3215.
doi: 10.1021/acs.orglett.7b01334 pmid: 28541046 |
| [46] |
Supranovich V. I.; Levin V. V.; Dilman A. D. Beilstein J. Org. Chem. 2020, 16, 1550.
doi: 10.3762/bjoc.16.126 pmid: 32704320 |
| [47] |
(a) Liu L.; Aguilera M. C.; Lee W.; Youshaw C. R.; Neidig M. L.; Gutierrez O. Science 2021, 374, 432.
doi: 10.1126/science.abj6005 |
|
(b) Rentería-Gómez A.; Lee W.; Yin S.; Davis M.; Gogoi A. R.; Gutierrez O. ACS Catal. 2022, 12, 11547.
doi: 10.1021/acscatal.2c03498 |
|
| [48] |
Ellefsen J. D.; Miller S. J. J. Org. Chem. 2022, 87, 10250.
doi: 10.1021/acs.joc.2c01231 |
| [49] |
Choi K.; Mormino M. G.; Kalkman E. D.; Park J.; Hartwig J. F. Angew. Chem., Int. Ed. 2022, 61, e202208204.
|
| [1] | 冯莹珂, 王贺, 崔梦行, 孙然, 王欣, 陈阳, 李蕾. 可见光诱导的新型官能化芳基异腈化合物的二氟烷基化环化反应[J]. 有机化学, 2023, 43(8): 2913-2925. |
| [2] | 黄华, 李鑫, 苏建科, 宋秋玲. 二氟卡宾参与下从邻乙烯基苯胺出发构建3-取代吲哚酮类化合物[J]. 有机化学, 2023, 43(3): 1146-1156. |
| [3] | 赵金晓, 魏彤辉, 柯森, 李毅. 可见光催化合成二氟烷基取代的多环吲哚化合物[J]. 有机化学, 2023, 43(3): 1102-1114. |
| [4] | 陈祥, 欧阳文韬, 李潇, 何卫民. 可见光诱导有机光催化合成二氟乙基苯并噁嗪[J]. 有机化学, 2023, 43(12): 4213-4219. |
| [5] | 孙奇, 孙泽颖, 俞泽, 王光伟. 镍催化炔烃的立体选择性芳基-二氟烷基化反应[J]. 有机化学, 2022, 42(8): 2515-2520. |
| [6] | 郭檬檬, 于子伦, 陈玉兰, 葛丹华, 马猛涛, 沈志良, 褚雪强. 二氟烯醇硅醚作为含氟砌块在构建有机氟化物中的研究进展[J]. 有机化学, 2022, 42(11): 3562-3587. |
| [7] | 朱文庆, 许婷怡, 韩文勇. 二氟甲基重氮甲烷作为含氟砌块的应用研究进展[J]. 有机化学, 2021, 41(4): 1275-1287. |
| [8] | 潘军, 吴晶晶, 吴范宏. 多组分参与的氟烷基化反应研究进展[J]. 有机化学, 2021, 41(3): 983-1001. |
| [9] | 秦文兵, 陈嘉怡, 熊威, 刘国凯. 亲电二氟甲基试剂及其应用研究进展[J]. 有机化学, 2020, 40(10): 3177-3195. |
| [10] | 高兴, 何旭, 张新刚. 镍催化下一溴二氟甲烷对(杂)芳基溴代物的二氟甲基化反应[J]. 有机化学, 2019, 39(1): 215-222. |
| [11] | 王为强, 余秦伟, 张前, 李江伟, 惠丰, 杨建明, 吕剑. 二氟甲基化方法研究进展[J]. 有机化学, 2018, 38(7): 1569-1585. |
| [12] | 张霁, 金传飞, 张英俊. 含氟药物研究进展和芳(杂)环氟化及N(n=1,2,3)氟甲基化新方法[J]. 有机化学, 2014, 34(4): 662-680. |
| [13] | 陈庆云. 抑铬雾剂F-53的研制带动了有机氟化学的发展[J]. 有机化学, 2001, 21(11): 805-809. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||