有机化学 ›› 2021, Vol. 41 ›› Issue (4): 1572-1581.DOI: 10.6023/cjoc202101009 上一篇 下一篇
所属专题: 热点论文虚拟合集
研究论文
收稿日期:2021-01-05
修回日期:2021-01-25
发布日期:2021-02-22
通讯作者:
徐森苗
基金资助:
Luhua Liua,b, Rongrong Dua,b, Senmiao Xua,*( )
)
Received:2021-01-05
Revised:2021-01-25
Published:2021-02-22
Contact:
Senmiao Xu
About author:Supported by:文章分享
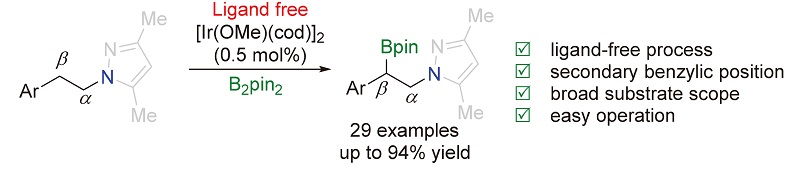
报道了无外加配体参与的以吡唑作为导向基团的铱催化的sp3碳氢键的区域选择性硼化反应. 在催化量的[Ir(OMe)(cod)]2存在下, 该反应能够顺利地将苄位的二级碳氢键转化成碳硼键. 该反应具有非常广谱的官能团兼容性, 能够以良好到优秀的产率生成相应的产物. 此外, 导向基团吡唑能够通过臭氧接转化成酰胺.
刘路华, 杜荣荣, 徐森苗. 无配体参与的铱催化的苄位二级碳氢键的硼化反应[J]. 有机化学, 2021, 41(4): 1572-1581.
Luhua Liu, Rongrong Du, Senmiao Xu. Ligand-Free Iridium-Catalyzed Borylation of Secondary Benzylic C—H Bonds[J]. Chinese Journal of Organic Chemistry, 2021, 41(4): 1572-1581.
| Entrya | Variation from standard conditions | Yieldb/% of 3 |
|---|---|---|
| 1 | None | 90 |
| 2 | [IrCl(cod)]2 instead of [IrOMe(cod)]2 | 81 |
| 3 | HBpin instead of B2pin2 | Trace |
| 4 | 1.0 equiv of B2pin2 was used | 50 |
| 5 | 2 mol% dtbpy was used | n.d. |
| 6 | 2 mol% P(C6F5)3 was used | 80 |
| 7 | 80 ℃ instead of 100 ℃ | 60 |
| 8 | 1,4-Dioxane instead of n-heptane | 33 |
| 9 | n-Hexane instead of n-heptane | 77 |
| Entrya | Variation from standard conditions | Yieldb/% of 3 |
|---|---|---|
| 1 | None | 90 |
| 2 | [IrCl(cod)]2 instead of [IrOMe(cod)]2 | 81 |
| 3 | HBpin instead of B2pin2 | Trace |
| 4 | 1.0 equiv of B2pin2 was used | 50 |
| 5 | 2 mol% dtbpy was used | n.d. |
| 6 | 2 mol% P(C6F5)3 was used | 80 |
| 7 | 80 ℃ instead of 100 ℃ | 60 |
| 8 | 1,4-Dioxane instead of n-heptane | 33 |
| 9 | n-Hexane instead of n-heptane | 77 |
| [1] |
(a) Cho, J.-Y.; Tse, M.K.; Holmes, D.; Maleczka, R.E.; Smith, M.R. Science 2002, 295,305.
doi: 10.1126/science.1067074 pmid: 11792205 |
|
(b) Ishiyama, T.; Takagi, J.; Ishida, K.; Miyaura, N.; Anastasi, N.R.; Hartwig, J.F. J. Am. Chem. Soc. 2002, 124,390.
doi: 10.1021/ja0173019 pmid: 11792205 |
|
|
(c) Mkhalid, I.A. I.; Barnard, J.H.; Marder, T.B.; Murphy, J.M.; Hartwig, J.F. Chem. Rev. 2010, 110,890.
pmid: 11792205 |
|
|
(d) Wang, M.; Shi, Z. Chem. Rev. 2020, 120,7438.
pmid: 11792205 |
|
| [2] |
Boller, T.M.; Murphy, J.M.; Hapke, M.; Ishiyama, T.; Miyaura, N.; Hartwig, J.F. J. Am. Chem. Soc. 2005, 127,14263.
pmid: 16218621 |
| [3] |
(a) Ros, A.; Fernández, R.; Lassaletta, J.M. Chem. Soc. Rev. 2014, 43,3229.
pmid: 24553599 |
|
(b) Hartwig, J.F. Chem. Soc. Rev. 2011, 40,1992.
pmid: 24553599 |
|
|
(c) Xu, L.; Wang, G.; Zhang, S.; Wang, H.; Wang, L.; Liu, L.; Jiao, J.; Li, P. Tetrahedron 2017, 73,7123.
pmid: 24553599 |
|
|
(d) Jin, J.; Xia, H.; Zhang, F.; Wang, Y. Chin. J. Org. Chem. 2020, 40,2185. (in Chinese)
pmid: 24553599 |
|
|
( 靳继康, 夏慧敏, 张凤莲, 汪义丰, 有机化学, 2020, 40,2185.)
pmid: 24553599 |
|
|
(e) Shi, D.; Wang, L.; Xia, C.; Liu, C. Chin. J. Org. Chem. 2020, 40,3605. (in Chinese)
pmid: 24553599 |
|
|
( 史敦发, 王露, 夏春谷, 刘超, 有机化学, 2020, 40,3605.)
pmid: 24553599 |
|
|
(f) He, H.; Jiang, Z. Chin. J. Org. Chem. 2020, 40,3483. (in Chinese)
pmid: 24553599 |
|
|
( 许荷欢, 江智勇, 有机化学, 2020, 40,3483.)
pmid: 24553599 |
|
|
(g) Zhu, S.; Chu, L. Chin. J. Org. Chem. 2020, 40,3980. (in Chinese)
pmid: 24553599 |
|
|
( 朱圣卿, 储玲玲, 有机化学, 2020, 40,3980.)
pmid: 24553599 |
|
| [4] |
Selected examples, see: (a) Thongpaen, J; Schmid, T. E.; Toupet, L.; Dorcet, V.; Mauduit, M.; Baslé, O. Chem. Commun. 2018, 54,8202.
pmid: 22369472 |
|
(b) Roering, A.J.; Hale, L.V. A.; Squier, P.A.; Ringgold, M.A.; Wiederspan, E.R.; Clark, T.B. Org. Lett. 2012, 14,3558.
pmid: 22369472 |
|
|
(c) Xu, F.; Duke, O.M.; Rojas, D.; Eichelberger, H.M.; Kim, R.S.; Clark, T.B.; Watson, D.A. J. Am. Chem. Soc. 2020, 142,11988.
pmid: 22369472 |
|
|
(d) Nakamura, T.; Suzuki, K.; Yamashita, M. J. Am. Chem. Soc. 2017, 139,17763.
pmid: 22369472 |
|
|
(e) Wang, G.; Liu, L.; Wang, H.; Ding, Y.-S.; Zhou, J.; Mao, S.; Li, P. J. Am. Chem. Soc. 2017, 139,91.
pmid: 22369472 |
|
|
(f) Ghaffari, B.; Preshlock, S.M.; Plattner, D.L.; Staples, R.J.; Maligres, P.E.; Krska, S.W.; Maleczka, R.E.; Smith, M.R. J. Am. Chem. Soc. 2014, 136,14345.
pmid: 22369472 |
|
|
(g) Wright, S.E.; Richardson-Solorzano, S.; Stewart, T.N.; Miller, C.D.; Morris, K.C.; Daley, C.J. A.; Clark, T.B. Angew. Chem. Int. Ed. 2019, 58,2834.
pmid: 22369472 |
|
|
(h) Kawamorita, S.; Miyazaki, T.; Ohmiya, H.; Iwai, T.; Sawamura, M. J. Am. Chem. Soc. 2011, 133,19310.
pmid: 22369472 |
|
|
(i) Kawamorita, S.; Ohmiya, H.; Hara, K.; Fukuoka, A.; Sawamura, M. J. Am. Chem. Soc. 2009, 131,5058.
pmid: 22369472 |
|
|
(j) Ros, A.; Estepa, B.; López-Rodríguez, R.; Álvarez, E.; Fernández, R.; Lassaletta, J.M. Angew. Chem. Int. Ed. 2011, 50,11724.
pmid: 22369472 |
|
|
(k) Ros, A.; López-Rodríguez, R.; Estepa, B.; Álvarez, E.; Fernández, R.; Lassaletta, J.M. J. Am. Chem. Soc. 2012, 134,4573.
pmid: 22369472 |
|
| [5] |
Selected examples, see: (a) Liu, L.; Wang, G.; Li, P. Org. Lett. 2017, 19,6132.
pmid: 28134536 |
|
(b) Yang, Y.; Gao, Q.; Xu, S. Adv. Synth. Catal. 2019, 361,858.
pmid: 28134536 |
|
|
(c) Yamamoto, T.; Ishibashi, A.; Suginome, M. Org. Lett. 2017, 19,886.
pmid: 28134536 |
|
|
(d) Crawford, K.M.; Ramseyer, T.R.; Daley, C.J. A.; Clark, T.B. Angew. Chem. Int. Ed. 2014, 53,7589.
pmid: 28134536 |
|
|
(e) Boebel, T.A.; Hartwig, J.F. J. Am. Chem. Soc. 2008, 130,7534.
pmid: 28134536 |
|
| [6] |
Selected examples, see: (a) Zeng, J; Naito, M.; Torigoe, T.; Yamanaka, M.; Kuninobu, Y. Org. Lett. 2020, 22,3485.
pmid: 32323992 |
|
(b) Bai, S.-T.; Bheeter, C.B.; Reek, J.N. H. Angew. Chem. Int. Ed. 2019, 58,13039.
pmid: 32323992 |
|
|
(c) Roosen, P.C.; Kallepalli, V.A.; Chattopadhyay, B.; Singleton, D.A.; Maleczka, R.E.; Smith, M.R. J. Am. Chem. Soc. 2012, 134,11350.
pmid: 32323992 |
|
|
(d) Preshlock, S.M.; Plattner, D.L.; Maligres, P.E.; Krska, S.W.; Maleczka, R.E.; Smith, M.R. Angew. Chem. Int. Ed. 2013, 52,12915.
pmid: 32323992 |
|
|
(e) Smith, M.R.; Bisht, R.; Haldar, C.; Pandey, G.; Dannatt, J.E.; Ghaffari, B.; Maleczka, R.E.; Chattopadhyay, B. ACS Catal. 2018, 8,6216.
pmid: 32323992 |
|
|
(f) Kuninobu, Y.; Ida, H.; Nishi, M.; Kanai, M. Nat. Chem. 2015, 7,712.
pmid: 32323992 |
|
| [7] |
Chattopadhyay, B.; Dannatt, J.E.; Andujar-De Sanctis, I.L.; Gore, K.A.; Maleczka, R.E.; Singleton, D.A.; Smith, M.R. J. Am. Chem. Soc. 2017, 139,7864.
|
| [8] |
(a) Mihai, M.T.; Williams, B.D.; Phipps, R.J. J. Am. Chem. Soc. 2019, 141,15477.
|
|
(b) Davis, H.J.; Genov, G.R.; Phipps, R.J. Angew. Chem. Int. Ed. 2017, 56,13351.
|
|
|
(c) Davis, H.J.; Mihai, M.T.; Phipps, R.J. J. Am. Chem. Soc. 2016, 138,12759.
|
|
| [9] |
Yang, L.; Semba, K.; Nakao, Y. Angew. Chem. Int. Ed. 2017, 56,4853.
|
| [10] |
(a) Liskey, C.W.; Hartwig, J.F. J. Am. Chem. Soc. 2012, 134,12422.
pmid: 23398347 |
|
(b) Oeschger, R.; Su, B.; Yu, I.; Ehinger, C.; Romero, E.; He, S.; Hartwig, J. Science 2020, 368,736.
pmid: 23398347 |
|
|
(c) Zhong, R.-L.; Sakaki, S. J. Am. Chem. Soc. 2019, 141,9854.
pmid: 23398347 |
|
|
(d) Larsen, M.A.; Oeschger, R.J.; Hartwig, J.F. ACS Catal. 2020, 10,3415.
pmid: 23398347 |
|
|
(e) Ohmura, T.; Torigoe, T.; Suginome, M. J. Am. Chem. Soc. 2012, 134,17416.
pmid: 23398347 |
|
|
(f) Kawamorita, S.; Murakami, R.; Iwai, T.; Sawamura, M. J. Am. Chem. Soc. 2013, 135,2947.
pmid: 23398347 |
|
| [11] |
Cho, S.H.; Hartwig, J.F. J. Am. Chem. Soc. 2013, 135,8157.
|
| [12] |
Kang, E.; Kim, H.T.; Joo, J.M. Org. Biomol. Chem. 2020, 18,6192.
pmid: 32705094 |
| [13] |
Boerth, J.A.; Hummel, J.R.; Ellman, J.A. Angew. Chem. Int. Ed. 2016, 55,12650.
|
| [14] |
(a) Gulia, N.; Daugulis, O. Angew. Chem. Int. Ed. 2017, 56,3630.
pmid: 29016134 |
|
(b) Yang, W.; Ye, S.; Fanning, D.; Coon, T.; Schmidt, Y.; Krenitsky, P.; Stamos, D.; Yu, J.-Q. Angew. Chem. Int. Ed. 2015, 54,2501.
pmid: 29016134 |
|
|
(c) Shabashov, D.; Daugulis, O. Org. Lett. 2005, 7,3657.
pmid: 29016134 |
|
|
(d) Hull, K.L.; Sanford, M.S. J. Am. Chem. Soc. 2007, 129,11904.
pmid: 29016134 |
|
|
(e) Shen, Y.; Cindy Lee, W.-C.; Gutierrez, D.A.; Li, J.J. J. Org. Chem. 2017, 82,11620.
pmid: 29016134 |
|
| [15] |
(a) Ackermann, L.; Althammer, A.; Born, R. Tetrahedron 2008, 64,6115.
pmid: 23534668 |
|
(b) Ackermann, L.; Althammer, A.; Born, R. Angew. Chem. Int. Ed. 2006, 45,2619.
pmid: 23534668 |
|
|
(c) Hofmann, N.; Ackermann, L. J. Am. Chem. Soc. 2013, 135,5877.
pmid: 23534668 |
|
|
(d) Wang, Y.; Liu, H.; Li, B.; Wang, B. Adv. Syn. Catal. 2019, 361,1564.
pmid: 23534668 |
|
| [16] |
(a) Yuan, C.; Tu, G.; Zhao, Y. Org. Lett. 2017, 19,356.
pmid: 31386754 |
|
(b) Li, T.; Liu, C.; Wu, S.; Chen, C.; Zhu, B. Org. Biomol. Chem. 2019, 17,7679.
pmid: 31386754 |
|
|
(c) Cai, H.; Thombal, R.S.; Li, X.; Lee, Y.R. Adv. Synth. Catal. 2019, 361,4022.
pmid: 31386754 |
|
|
(d) Kim, H.; Thombal, R.S.; Khanal, H.D.; Lee, Y.R. Chem. Commun. 2019, 55,13402.
pmid: 31386754 |
|
|
(e) Saitou, T.; Jin, Y.; Isobe, K.; Suga, T.; Takaya, J.; Iwasawa, N. Chem. Asian J. 2020, 15,1941.
pmid: 31386754 |
|
| [17] |
Gao, P.; Guo, W.; Xue, J.; Zhao, Y.; Yuan, Y.; Xia, Y.; Shi, Z. J. Am. Chem. Soc. 2015, 137,12231.
pmid: 26348796 |
| [18] |
(a) Wang, Y.; Liu, H.; Li, B.; Wang, B. Adv. Synth. Catal. 2019, 361,1564.
|
|
(b) Botla, V.; Akudari, A.; Malapaka, C. Tetrahedron Lett. 2019, 60,115.
|
|
|
(c) Lee, W.-C. C.; Shen, Y.; Gutierrez, D.A.; Li, J.J. Org. Lett. 2016, 18,2660.
|
|
| [19] |
Crystallographic data for 13 could be found in the Supporting Information. CCDC 2031418 (13) contains the supplementary crystallographic data for this paper. The data can be obtained free of charge from The Cambridge Crystallographic Data Center via .
|
| [20] |
Hu, N.; Zhao, G.; Zhang, Y.; Liu, X.; Li, G.; Tang, W. J. Am. Chem. Soc. 2015, 137,6746.
|
| [21] |
Liu, Z.; Ni, H.-Q.; Zeng, T.; Engle, K.M. J. Am. Chem. Soc. 2018, 140,3223.
|
| [22] |
Kawamorita, S.; Miyazaki, T.; Iwai, T.; Ohmiya, H.; Sawamura, M. J. Am. Chem. Soc. 2012, 134,12924.
|
| [23] |
Bonet, A.; Odachowski, M.; Leonori, D.; Essafi, S.; Aggarwal, V.K. Nat. Chem. 2014, 6,584.
|
| [24] |
Kremsner, J.M.; Kappe, C.O. J. Org. Chem. 2006, 71,4651.
pmid: 16749800 |
| [1] | 李思达, 崔鑫, 舒兴中, 吴立朋. 钛催化的烯烃制备1,1-二硼化合物[J]. 有机化学, 2024, 44(2): 631-637. |
| [2] | 王文芳. 过渡金属催化不对称C—H硼化反应研究进展[J]. 有机化学, 2023, 43(9): 3146-3166. |
| [3] | 陈祖佳, 宇世伟, 周永军, 李焕清, 邱琪雯, 李妙欣, 汪朝阳. BF3•OEt2作为催化剂与合成子在有机合成中的应用进展[J]. 有机化学, 2023, 43(9): 3107-3118. |
| [4] | 关丽, 周艳艳, 毛永爆, 付恺森, 关文惠, 付义乐. 甲川链修饰菁染料的合成研究进展[J]. 有机化学, 2023, 43(8): 2682-2698. |
| [5] | 秦玉承, 徐良轩, 徐佳能, 刘超. 1,2-迁移促进的苄基季铵盐硼化反应研究[J]. 有机化学, 2023, 43(5): 1868-1874. |
| [6] | 梁凯淳, 白科研, 戴雷, 刘源, 叶泽聪, 霍延平. 基于四氢喹啉的多重共振热活化延迟荧光材料的设计、合成及电致发光性能研究[J]. 有机化学, 2023, 43(5): 1799-1807. |
| [7] | 徐茂财, 田佳壮, 杨艳华, 苟高章, 李福敏, 邵林, 池可心. 一种三苯胺基二氟硼发光化合物的力致可逆荧光变色及数据安全保护研究[J]. 有机化学, 2023, 43(5): 1824-1831. |
| [8] | 杜琳琳, 张华. 芳烃与烷烃化合物参与的光化学与电化学硼化反应[J]. 有机化学, 2023, 43(5): 1726-1741. |
| [9] | 徐晓阳, 刘美艳, 李成龙, 刘旭光. 1,2-硼氮杂芳烃在中国的研究进展[J]. 有机化学, 2023, 43(5): 1611-1644. |
| [10] | 陈志豪, 范奇, 尹标林, 李清江, 王洪根. α-硼取代羰基类化合物的合成进展[J]. 有机化学, 2023, 43(5): 1706-1712. |
| [11] | 蒋旺, 史壮志. 芳烃间/对位选择性碳氢硼化反应研究进展[J]. 有机化学, 2023, 43(5): 1691-1705. |
| [12] | 党燕, 贾朝红, 王亚兰, 王丽, 李亚飞, 李亚红. 含吡咯基配体的锌、锂和镁配合物的合成与表征及其对芳基碘代物的硼化反应和醛、酮的硼氢化反应的催化作用[J]. 有机化学, 2023, 43(3): 1124-1135. |
| [13] | 宋树勇, 徐森苗. 三氟甲基烯烃的选择性C-F键活化最新进展[J]. 有机化学, 2023, 43(2): 411-425. |
| [14] | 陈宇亮, 贺凤开, 王思云, 贾鼎成, 刘亚群, 黄毅勇. 手性磷酸催化α-全碳季碳醛的不对称烯丙基化动力学拆分[J]. 有机化学, 2023, 43(12): 4294-4302. |
| [15] | 刘鹏, 钟富明, 廖礼豪, 谭伟强, 赵晓丹. 炔烃参与的去芳构化反应构建螺环己二烯酮类化合物的研究进展[J]. 有机化学, 2023, 43(12): 4019-4035. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||