有机化学 ›› 2021, Vol. 41 ›› Issue (9): 3550-3559.DOI: 10.6023/cjoc202101014 上一篇 下一篇
研究论文
刘新, 许润梅, 王淋, 刘雅雪, 陈志豪, 秦巍, 田玉顺*( )
)
收稿日期:2021-01-07
修回日期:2021-03-15
发布日期:2021-07-19
通讯作者:
田玉顺
作者简介:基金资助:
Xin Liu, Runmei Xu, Lin Wang, Yaxue Liu, Zhihao Chen, Wei Qin, Yushun Tian( )
)
Received:2021-01-07
Revised:2021-03-15
Published:2021-07-19
Contact:
Yushun Tian
About author:Supported by:文章分享

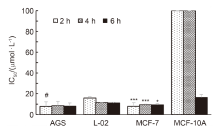
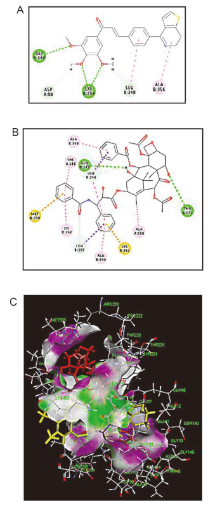
以2-噻吩乙胺与自制的查尔酮酸进行酰化反应得到酰胺类中间体5a~5j, 经Bischer-Napieralski环合反应合成了10个未见报道的二氢噻吩并吡啶-查尔酮衍生物6a~6j, 再经去氢反应获得2个噻吩并吡啶-查尔酮衍生物7a和7b. 通过噻唑蓝(MTT)法对11种细胞进行体外抗癌活性及安全性测试. 结果表明, 化合物6a (p-F)、6d (o-Br)和6h (m-OCH3)对HeLa、SGC-7901细胞的抗癌活性优于紫杉醇. 当短时间处理(<4 h)时, 6j (3,4,5-OCH3)在不影响正常细胞MCF-10A的情况下对癌细胞MCF-7显示强效抗癌效果, 值得进一步研究和开发.
刘新, 许润梅, 王淋, 刘雅雪, 陈志豪, 秦巍, 田玉顺. 二氢噻吩并吡啶-查尔酮衍生物的合成及其体外抗肿瘤活性研究[J]. 有机化学, 2021, 41(9): 3550-3559.
Xin Liu, Runmei Xu, Lin Wang, Yaxue Liu, Zhihao Chen, Wei Qin, Yushun Tian. Synthesis and Evaluation in vitro of Dihydrothiophenopyridine-Chalcone Derivatives as Anticancer Activity Agents[J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3550-3559.
| Compd. | R | IC50/(µmol•L–1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BEL-7402 | HepG2 | HCT116 | A549 | MGC-803 | SGC-7901 | AGS | MCF-7 | MCF-10A | HeLa | L-02 | ||
| 3a | p-F | 31.7±5.4 | >100 | 10.7±2.2 | >100 | 18.9±2.1 | 22.0±1.3 | 22.7±7.8 | 40.6±9.2 | 32.8±2.8 | 39.1±12.1 | 21.2±5.7 |
| 3b | p-Cl | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 3c | p-H | 14.3±3.4 | >100 | 21.0±0.5 | >100 | >100 | >100 | 32.3±1.4 | 24.1±0.4 | 47.4±4.6 | 35.3±2.3 | 27.1±1.6 |
| 3d | o-Br | 25.8±3.3 | 94.3±5.1 | 8.63±2.1 | >100 | 15.4±6.1 | 26.6±4.6 | 22.8±7.3 | 45.1±5.7 | 37.2±6.9 | 69.5±8.8 | 23.4±2.8 |
| 3e | m-Br | 5.9±0.03 | 11.7±4.7 | 3.0±2.0 | 20.2±4.3 | 5.3±0.8 | 3.2±0.8 | 5.3±3.5 | 13.6±2.3 | 8.2±2.3 | 23.5±8.8 | 5.3±0.3 |
| 3f | p-Br | 10.3±0.1 | 35.5±13.7 | 3.5±1.9 | 57.2±4.5 | 9.9±1.2 | 3.3±1.9 | 12.8±3.0 | 17.2±4.5 | 16.1±2.7 | 15.0±4.9 | 11.4±3.4 |
| 3g | p-CH3 | 16.0±2.5 | 42.0±3.2 | 8.0±1.9 | 50.8±3.0 | 15.1±1.0 | 12.5±1.5 | 24.7±7.0 | 27.4±3.9 | 28.9±5.5 | 36.3±7.9 | 15.6±1.8 |
| 3h | m-OCH3 | 23.6±2.7 | 44.5±15.4 | 7.2±0.9 | >100 | 15.6±0.8 | 11.7±1.1 | 36.7±10.2 | 28.0±3.9 | 28.8±7.4 | 55.4±8.9 | 18.8±2.1 |
| 3i | p-OCH3 | 12.3±3.1 | 89.6±0.4 | 6.4±3.2 | >100 | 8.5±0.8 | 10.7±2.2 | 16.9±1.5 | >100 | 51.1±5.7 | 42.6±8.0 | 7.5±1.7 |
| 3j | 3,4,5-(OCH3)3 | 8.0±1.4 | >100 | >100 | >100 | 11.0±1.4 | >100 | 11.3±3.4 | 21.5±4.3 | 38.8±11.8 | 39.4±1.2 | 5.8±2.6 |
| Taxol | 0.09±0.02 | 0.94±0.03 | 0.03±0.01 | 0.44±0.02 | 0.06±0.01 | >100 | 0.02±0.01 | 0.08±0.01 | 0.18±0.02 | 13.0±0.4 | >100 | |
| Compd. | R | IC50/(µmol•L–1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BEL-7402 | HepG2 | HCT116 | A549 | MGC-803 | SGC-7901 | AGS | MCF-7 | MCF-10A | HeLa | L-02 | ||
| 3a | p-F | 31.7±5.4 | >100 | 10.7±2.2 | >100 | 18.9±2.1 | 22.0±1.3 | 22.7±7.8 | 40.6±9.2 | 32.8±2.8 | 39.1±12.1 | 21.2±5.7 |
| 3b | p-Cl | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 3c | p-H | 14.3±3.4 | >100 | 21.0±0.5 | >100 | >100 | >100 | 32.3±1.4 | 24.1±0.4 | 47.4±4.6 | 35.3±2.3 | 27.1±1.6 |
| 3d | o-Br | 25.8±3.3 | 94.3±5.1 | 8.63±2.1 | >100 | 15.4±6.1 | 26.6±4.6 | 22.8±7.3 | 45.1±5.7 | 37.2±6.9 | 69.5±8.8 | 23.4±2.8 |
| 3e | m-Br | 5.9±0.03 | 11.7±4.7 | 3.0±2.0 | 20.2±4.3 | 5.3±0.8 | 3.2±0.8 | 5.3±3.5 | 13.6±2.3 | 8.2±2.3 | 23.5±8.8 | 5.3±0.3 |
| 3f | p-Br | 10.3±0.1 | 35.5±13.7 | 3.5±1.9 | 57.2±4.5 | 9.9±1.2 | 3.3±1.9 | 12.8±3.0 | 17.2±4.5 | 16.1±2.7 | 15.0±4.9 | 11.4±3.4 |
| 3g | p-CH3 | 16.0±2.5 | 42.0±3.2 | 8.0±1.9 | 50.8±3.0 | 15.1±1.0 | 12.5±1.5 | 24.7±7.0 | 27.4±3.9 | 28.9±5.5 | 36.3±7.9 | 15.6±1.8 |
| 3h | m-OCH3 | 23.6±2.7 | 44.5±15.4 | 7.2±0.9 | >100 | 15.6±0.8 | 11.7±1.1 | 36.7±10.2 | 28.0±3.9 | 28.8±7.4 | 55.4±8.9 | 18.8±2.1 |
| 3i | p-OCH3 | 12.3±3.1 | 89.6±0.4 | 6.4±3.2 | >100 | 8.5±0.8 | 10.7±2.2 | 16.9±1.5 | >100 | 51.1±5.7 | 42.6±8.0 | 7.5±1.7 |
| 3j | 3,4,5-(OCH3)3 | 8.0±1.4 | >100 | >100 | >100 | 11.0±1.4 | >100 | 11.3±3.4 | 21.5±4.3 | 38.8±11.8 | 39.4±1.2 | 5.8±2.6 |
| Taxol | 0.09±0.02 | 0.94±0.03 | 0.03±0.01 | 0.44±0.02 | 0.06±0.01 | >100 | 0.02±0.01 | 0.08±0.01 | 0.18±0.02 | 13.0±0.4 | >100 | |
| Compd. | R | IC50/(µmol•L–1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BEL-7402 | HepG2 | HCT116 | A549 | MGC-803 | SGC-7901 | AGS | MCF-7 | MCF-10A | HeLa | L-02 | ||
| 6a | p-F | 6.0±0.2 | 17.8±2.6 | 7.6±0.5 | 23.6±4.9 | 6.6±1.1 | 6.0±0.3 | 8.3±2.6 | 9.0±3.0 | 8.5±2.8 | 10.5±2.6 | 5.6±1.6 |
| 6b | p-Cl | 11.0±0.4 | >100 | 10.1±4.1 | 16.5±2.6 | 13.8±4.0 | >100 | 11.4±1.4 | 17.8±0.7 | 13.7±5.0 | 28.9±11.3 | 15.7±1.1 |
| 6c | p-H | 8.7±1.0 | >100 | 11.8±2.8 | 18.3±9.7 | 14.3±2.1 | 15.1±6.7 | 10.4±7.6 | 6.40±1.3 | 17.9±4.0 | 19.4±2.3 | 11.3±2.8 |
| 6d | o-Br | 3.8±4.8 | 15.6±0.3 | 5.1±0.5 | 20.2±3.5 | 3.6±1.7 | 6.7±0.03 | 5.7±2.5 | 10.2±1.4 | 7.6±1.2 | 10.9±5.6 | 6.9±1.7 |
| 6e | m-Br | 5.9±2.9 | 54.5±31.2 | 6.5±2.4 | 20.7±1.1 | 14.7±5.1 | 18.3±1.7 | 24.3±5.6 | 14.1±5.7 | 7.4±2.2 | 13.9±5.6 | 7.2±3.2 |
| 6f | p-Br | >100 | >100 | 5.2±2.1 | >100 | 15.7±0.4 | 17.3±6.5 | 37.0±12.7 | >100 | >100 | 46.1±1.6 | 41.1±2.5 |
| 6g | p-CH3 | 21.8±3.2 | >100 | 9.3±1.3 | 26.5±5.1 | 3.4±2.0 | 8.2±0.2 | 28.7±8.2 | 15.1±3.5 | 20.3±11.7 | 15.2±4.0 | 14.9±6.3 |
| 6h | m-OCH3 | 5.0±3.7 | 19.2±2.9 | 6.1±1.3 | 15.9±5.7 | 5.6±0.7 | 9.0±1.0 | 5.9±4.6 | 12.3±4.1 | 10.5±5.1 | 7.5±1.2 | 6.1±5.1 |
| 6i | p-OCH3 | 24.4±4.9 | >100 | 24.0±3.1 | 23.5±6.8 | 8.9±2.4 | 11.0±0.4 | 17.7±8.0 | 24.7±7.8 | >100 | 21.8±1.5 | 11.0±0.7 |
| 6j | 3,4,5-(OCH3)3 | 6.1±1.3 | >100 | 4.9±1.7 | 10.9±9.2 | 6.4±0.5 | >100 | 1.3±0.1 | 15.0±5.0 | 16.8±4.3 | 18.5±9.7 | 8.2±2.0 |
| 7a | p-F | 4.5±0.1 | >100 | 17.3±3.2 | 36.2±2.8 | 12.3±0.9 | 24.1±6.4 | 14.5±3.5 | 6.5±1.6 | 27.6±3.6 | 24.6±4.5 | 10.7±5.9 |
| 7b | p-Cl | 62.9±1.0 | >100 | 21.0±0.5 | >100 | >100 | 75.4±11.8 | 48.9±0.4 | 56.4±10.3 | >100 | >100 | 32.5±0.6 |
| Taxol | 0.09±0.02 | 0.94±0.03 | 0.03±0.01 | 0.44±0.02 | 0.06±0.01 | >100 | 0.02±0.01 | 0.08±0.01 | 0.18±0.02 | 13.0±0.4 | >100 | |
| Compd. | R | IC50/(µmol•L–1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BEL-7402 | HepG2 | HCT116 | A549 | MGC-803 | SGC-7901 | AGS | MCF-7 | MCF-10A | HeLa | L-02 | ||
| 6a | p-F | 6.0±0.2 | 17.8±2.6 | 7.6±0.5 | 23.6±4.9 | 6.6±1.1 | 6.0±0.3 | 8.3±2.6 | 9.0±3.0 | 8.5±2.8 | 10.5±2.6 | 5.6±1.6 |
| 6b | p-Cl | 11.0±0.4 | >100 | 10.1±4.1 | 16.5±2.6 | 13.8±4.0 | >100 | 11.4±1.4 | 17.8±0.7 | 13.7±5.0 | 28.9±11.3 | 15.7±1.1 |
| 6c | p-H | 8.7±1.0 | >100 | 11.8±2.8 | 18.3±9.7 | 14.3±2.1 | 15.1±6.7 | 10.4±7.6 | 6.40±1.3 | 17.9±4.0 | 19.4±2.3 | 11.3±2.8 |
| 6d | o-Br | 3.8±4.8 | 15.6±0.3 | 5.1±0.5 | 20.2±3.5 | 3.6±1.7 | 6.7±0.03 | 5.7±2.5 | 10.2±1.4 | 7.6±1.2 | 10.9±5.6 | 6.9±1.7 |
| 6e | m-Br | 5.9±2.9 | 54.5±31.2 | 6.5±2.4 | 20.7±1.1 | 14.7±5.1 | 18.3±1.7 | 24.3±5.6 | 14.1±5.7 | 7.4±2.2 | 13.9±5.6 | 7.2±3.2 |
| 6f | p-Br | >100 | >100 | 5.2±2.1 | >100 | 15.7±0.4 | 17.3±6.5 | 37.0±12.7 | >100 | >100 | 46.1±1.6 | 41.1±2.5 |
| 6g | p-CH3 | 21.8±3.2 | >100 | 9.3±1.3 | 26.5±5.1 | 3.4±2.0 | 8.2±0.2 | 28.7±8.2 | 15.1±3.5 | 20.3±11.7 | 15.2±4.0 | 14.9±6.3 |
| 6h | m-OCH3 | 5.0±3.7 | 19.2±2.9 | 6.1±1.3 | 15.9±5.7 | 5.6±0.7 | 9.0±1.0 | 5.9±4.6 | 12.3±4.1 | 10.5±5.1 | 7.5±1.2 | 6.1±5.1 |
| 6i | p-OCH3 | 24.4±4.9 | >100 | 24.0±3.1 | 23.5±6.8 | 8.9±2.4 | 11.0±0.4 | 17.7±8.0 | 24.7±7.8 | >100 | 21.8±1.5 | 11.0±0.7 |
| 6j | 3,4,5-(OCH3)3 | 6.1±1.3 | >100 | 4.9±1.7 | 10.9±9.2 | 6.4±0.5 | >100 | 1.3±0.1 | 15.0±5.0 | 16.8±4.3 | 18.5±9.7 | 8.2±2.0 |
| 7a | p-F | 4.5±0.1 | >100 | 17.3±3.2 | 36.2±2.8 | 12.3±0.9 | 24.1±6.4 | 14.5±3.5 | 6.5±1.6 | 27.6±3.6 | 24.6±4.5 | 10.7±5.9 |
| 7b | p-Cl | 62.9±1.0 | >100 | 21.0±0.5 | >100 | >100 | 75.4±11.8 | 48.9±0.4 | 56.4±10.3 | >100 | >100 | 32.5±0.6 |
| Taxol | 0.09±0.02 | 0.94±0.03 | 0.03±0.01 | 0.44±0.02 | 0.06±0.01 | >100 | 0.02±0.01 | 0.08±0.01 | 0.18±0.02 | 13.0±0.4 | >100 | |


| Compd. | R | m.p./℃ | Yield/% |
|---|---|---|---|
| 3a | p-H | 212~213 | 61.8 |
| 3b | p-F | 255~256 | 79.7 |
| 3c | p-Cl | 259~260 | 95.3 |
| 3d | o-Br | 232~233 | 83.9 |
| 3e | m-Br | 206~207 | 74.7 |
| 3f | p-Br | 246~247 | 77.3 |
| 3g | p-CH3 | 242~243 | 81.9 |
| 3h | m-OCH3 | 101~102 | 66.3 |
| 3i | p-OCH3 | 224~226 | 84.6 |
| 3j | 3,4,5-(OCH3)3 | 223~224 | 61.7 |
| Compd. | R | m.p./℃ | Yield/% |
|---|---|---|---|
| 3a | p-H | 212~213 | 61.8 |
| 3b | p-F | 255~256 | 79.7 |
| 3c | p-Cl | 259~260 | 95.3 |
| 3d | o-Br | 232~233 | 83.9 |
| 3e | m-Br | 206~207 | 74.7 |
| 3f | p-Br | 246~247 | 77.3 |
| 3g | p-CH3 | 242~243 | 81.9 |
| 3h | m-OCH3 | 101~102 | 66.3 |
| 3i | p-OCH3 | 224~226 | 84.6 |
| 3j | 3,4,5-(OCH3)3 | 223~224 | 61.7 |
| [1] |
Khalifa, S. A. M.; Elias, N.; Farag, M. A.; Chen, L.; Saeed, A.; Hegazy, M. F.; Moustafa, M. S.; Abd, E. A.; Al-Mousawi, S. M.; Musharraf, S. G.; Chang, F. R.; Iwasaki, A.; Suenaga, K.; Alajlani, M.; Göransson, U.; El-Seedi, H. R. Mar. Drugs 2019, 17, 491.
doi: 10.3390/md17090491 |
| [2] |
Fujita, M.; Nakao, Y.; Matsunaga, S.; Seiki, M.; Itoh, Y.; Yamashita, J.; Van Soest, R. W. M.; Fusetani, N. J. Am. Chem. Soc. 2003, 125, 1570.
|
| [3] |
Bickmeyer, U.; Thoms, S.; Koch, F.; Mukagatare, L. P.; Silalahi, R.; Sartoris, F. J. PLoS One 2019, 14, e0213771.
doi: 10.1371/journal.pone.0213771 |
| [4] |
Iwata, T.; Otsuka, S.; Tsubokura, K.; Kurbangalieva, A.; Arai, D.; Fukase, K.; Nakao, Y.; Tanaka, K. Chem.-Eur. J. 2016, 22: 14707.
|
| [5] |
Ma, Y.; Nam, S.; Jove, R.; Yakushijin, K.; Horne, D. Bioorg. Med. Chem. Lett. 2010, 20, 84.
|
| [6] |
Srivastava, B. K.; Solanki, M.; Mishra, B.; Soni, R.; Jayadev, S.; Valani, D.; Jain, M.; Patel, P. R. Bioorg. Med. Chem. Lett. 2007, 17, 1924.
pmid: 17276057 |
| [7] |
Tang, J.; Lackey, K. E.; Dickerson, S. H. Bioorg. Med. Chem. Lett. 2013, 23, 66.
doi: 10.1016/j.bmcl.2012.11.020 pmid: 23218715 |
| [8] |
Liu, H.; Li, Y.; Wang, X. Y.; Wang, B.; He, H. Y.; Liu, J. Y.; Xiang, M. L.; He, J.; Wu, X. H.; Yang, L. Bioorg. Med. Chem. Lett. 2013, 23, 2349.
doi: 10.1016/j.bmcl.2013.02.059 |
| [9] |
Zhou, S. B; Duan, Y. N.; Wang, J.; Zhang, J.; Sun, H. F; Jiang, H. W; Gu, Z. N; Tong, J. H; Li, J. Y; Li, J.; Liu, H. Eur. J. Med. Chem. 2017, 140, 448.
doi: 10.1016/j.ejmech.2017.09.012 |
| [10] |
Kühn, F. J. P.; Kuhn, C.; Lückhoff, A. J. Biol. Chem. 2019, 16, 4102.
|
| [11] |
Wei, K. R.; Peng, X. B.; Liang, Z. H.; Cen, H. S. China Cancer 2015, 24, 621. (in Chinese).
|
|
( 魏矿荣, 彭侠彪, 梁智恒, 岑惠珊, 中国肿瘤, 2015, 24, 621.)
|
|
| [12] |
Zeng, X. X.; Zheng, R. L.; Zhou, T.; He, H. Y.; Liu, J. Y.; Zheng, Y.; Tong, A. P.; Xiang, M. L.; Song, X. R.; Yang, S. Y.; Yu, L. T.; Wei, Y. Q.; Zhao, Y. L.; Yang, L. Bioorg. Med. Chem. Lett. 2010, 20, 6282.
doi: 10.1016/j.bmcl.2010.08.088 |
| [13] |
Tian, L.; Wu, G. R.; Wang, Y. Northwest Pharm. J. 2007, 22, 218. (in Chinese).
|
|
( 田兰, 吴桂荣, 王岩, 西北药学杂志, 2007, 22, 218.)
|
|
| [14] |
Wang, Y. H.; Bai, H.; Dou, D. Q.; Pei, Y. P.; Chen, Y. J.; Li, W.; Kazuo, K.; Xing, S. R; Tamots, N. Northwest Pharm. J. 2004, 19, 253. (in Chinese).
|
|
( 王英华, 白虹, 窦德强, 裴玉萍, 陈英杰, 李巍, 小池一男, 邢世瑞, 二阶堂保, 西北药学杂志, 2004, 19, 253.)
|
|
| [15] |
Jandial, D. D.; Krill, L. S.; Chen, L.; Wu, C.; Ke, Y.; Xie, J.; Hoang, B. H.; Zi, X. Molecules 2017, 22, 462.
doi: 10.3390/molecules22030462 |
| [16] |
Kurt, B. Z.; Kandas, N. O.; Dag, A.; Sonmez, F.; Kucukislamoglu, M. Arab. J. Chem. 2020, 13, 1120.
doi: 10.1016/j.arabjc.2017.10.001 |
| [17] |
Sheng, Q. E.; Zhao, W. Q.; Zeng, M.; Xie, Z. B.; Xia, Y. P.; Cui, D. M. Chin. J. Org. Chem. 2019, 39, 703. (in Chinese).
doi: 10.6023/cjoc201808037 |
|
( 盛琦威, 赵婉秋, 曾明, 谢中袍, 夏雅平, 崔冬梅, 有机化学, 2019, 39, 703.)
|
|
| [18] |
Roussaki, M.; Hall, B.; Lima, S. C.; Da, S. A. C.; Wilkinson, S.; Detsi, A. Bioorg. Med. Chem. Lett. 2013, 23, 6436.
doi: 10.1016/j.bmcl.2013.09.047 pmid: 24119553 |
| [19] |
Dong, L. R.; Hu, D. Y.; Wu, Z. X.; Chen, J. X.; Song, B. A. Chin. Chem. Lett. 2017, 28, 1566.
doi: 10.1016/j.cclet.2017.03.013 |
| [20] |
Abdullah, M. I.; Mahmood, A.; Madni, M.; Masood, S.; Kashif, M. Bioorg. Chem. 2014, 54, 31.
doi: 10.1016/j.bioorg.2014.03.006 |
| [21] |
Anuradha, V.; Pullela, V.; Srinivas, R.; Ranga, R. K.; Manjulatha, M. G.; Purohit, J.; Adhusudana, R. Bioorg. Med. Chem. 2006, 14, 6820.
doi: 10.1016/j.bmc.2006.06.048 |
| [22] |
Zhao, L.; Jin, H.; Sun, L.; Piao, H.; Quan, Z. Bioorg. Med. Chem. Lett. 2005, 15, 5027.
doi: 10.1016/j.bmcl.2005.08.039 |
| [23] |
Zhao, L. J.; Shi, Y.; Liu, W.; Lin, X. M.; Sun, T. M.; Li, Y. L. Chin. J. Med. Chem. 2010, 20, 163. (in Chinese).
|
|
( 赵乐晶, 石玉, 刘巍, 林晓明, 孙铁民, 李祎亮, 中国药物化学杂志, 2010, 20, 163.)
|
|
| [24] |
Tamayo, N. A.; Bo, Y.; Gore, V.; Ma, V.; Nishimura, N.; Tang, P.; Deng, H.; Klionsky, L.; Lehto, S. G.; Wang, W.; Youngblood, B.; Chen, J.; Correll, T. L.; Bartberger, M. D.; Gavva, N. R.; Norman, M. H. J. Med. Chem. 2012, 55, 1594.
|
| [25] |
Shumaila, A. M. A.; Puranik, V. G.; Kusurkar, R. S. Tetrahedron 2011, 67, 936.
doi: 10.1016/j.tet.2010.12.003 |
| [26] |
Yan, Y. K.; Xu, Q.; Gao, Y.; Liu, H.; Tang, X. R. Chin. J. Org. Chem. 2018, 38, 1763. (in Chinese).
doi: 10.6023/cjoc201712027 |
|
( 严映坤, 徐侨, 高扬, 刘辉, 唐孝荣, 有机化学, 2018, 38, 1763.)
|
|
| [27] |
Zhang, J.; Li, F.; Li, Y.; Guo, Y.; Wang, J.; Zhang, S. Med. Chem. 2014, 10, 277.
doi: 10.2174/157340641003140304144930 |
| [28] |
Liu, Y. N.; Wang, J. J.; Ji, Y. T.; Zhao, G. D.; Tang, L. Q.; Zhang, C. M.; Guo, X. L.; Liu, Z. P. J. Med. Chem. 2016, 59, 5341.
doi: 10.1021/acs.jmedchem.6b00071 |
| [29] |
Farooq, S.; Ngaini, Z.; Mortadza, N. A. Bull. Korean Chem. Soc. 2020, 41, 920.
|
| [30] |
de Souza, A. C. A.; Mori, M.; Sens, L.; Rocha, R. F.; Tizziani, T.; de Souza, L. F. S.; Domeneghini Chiaradia-Delatorre, L.; Botta, M.; Nunes, R. J.; Terenzi, H.; Menegatti, A. C. O. Bioorg. Med. Chem. Lett. 2007, 30, 127350.
doi: 10.1016/j.bmcl.2020.127350 |
| [31] |
Jakovljevic, K.; Joksovic, M.; Matić, I. Z.; Petrovic, N.; Stanojkovic, T.; Sladić, D.; Vujcic, M.; Janovic, B.; Joksovic, L.; Trifunovic, S.; Marković, V. Med. Chem. Comm. 2018, 9, 1682.
|
| [32] |
Parveen, Z.; Brunhofer, G.; Jabeen, I.; Erker, T.; Chiba, P.; Ecker, G. F. Bioorg. Med. Chem. Lett. 2014, 22, 2314.
|
| [1] | 付雅彤, 孙超凡, 张丹, 金成国, 陆居有. 巢式-碳硼烷硼氢键官能化反应研究进展[J]. 有机化学, 2024, 44(2): 438-447. |
| [2] | 张剑, 梁万洁, 杨艺, 闫法超, 刘会. 联烯胺化合物的区域选择性双官能团化[J]. 有机化学, 2024, 44(2): 335-348. |
| [3] | 陈宛婷, 钟雄威, 邢佳乐, 吴昌书, 高杨. C—N轴手性化合物的不对称催化合成研究进展[J]. 有机化学, 2024, 44(2): 349-377. |
| [4] | 张莹珍, 江丹丹, 李娟华, 王菁菁, 刘昆明, 刘晋彪. 高选择性硒代半胱氨酸荧光探针的构建策略及成像[J]. 有机化学, 2024, 44(1): 41-53. |
| [5] | 王婧怡, 刘金羽, 陈东升, 陈华燕, 谢欣, 南发俊. 新型选择性半胱氨酰白三烯受体1 (CysLT1R)拮抗剂的设计合成及构效关系研究[J]. 有机化学, 2024, 44(1): 259-276. |
| [6] | 张俊杰, 徐学涛. (S)-(–)-Xylopinine和(S)-(+)-Laudanosine的不对称合成[J]. 有机化学, 2023, 43(9): 3297-3303. |
| [7] | 王兢睿, 冯永奎, 王能中, 黄年玉, 姚辉. 钯催化立体选择性合成硝基烷类β-碳糖苷[J]. 有机化学, 2023, 43(9): 3216-3225. |
| [8] | 范威. O2促进下五元环烯胺的C—H亚胺化[J]. 有机化学, 2023, 43(7): 2492-2498. |
| [9] | 户晓兢, 郭斐翔, 朱润青, 周柄棋, 张涛, 房立真. 对烷氧基酚的合成及其去芳构化后的合成应用[J]. 有机化学, 2023, 43(6): 2239-2244. |
| [10] | 蒋旺, 史壮志. 芳烃间/对位选择性碳氢硼化反应研究进展[J]. 有机化学, 2023, 43(5): 1691-1705. |
| [11] | 钟玉梅, 邹小颖, 卓小丫, 王逸涵, 申佳奕, 郑绿茵, 郭维. 4-氧代-2-亚胺基噻唑烷-5-亚基乙酸乙酯类化合物的设计、合成及抗癌活性[J]. 有机化学, 2023, 43(4): 1452-1461. |
| [12] | 纪健, 刘进华, 管丛, 陈绪文, 赵芸, 刘顺英. 原位生成的磺酸催化N-磺酰基-1,2,3-三氮唑与醇偶联高区域选择性合成N2-取代1,2,3-三氮唑[J]. 有机化学, 2023, 43(3): 1168-1176. |
| [13] | 向勋, 何照林, 董秀琴. 钯和手性磷酸协同催化高效构建手性分子的研究进展[J]. 有机化学, 2023, 43(3): 791-808. |
| [14] | 郭萍, 周勇, 赵杰. 多取代烯烃的Z∶E高选择性合成制备[J]. 有机化学, 2023, 43(3): 855-872. |
| [15] | 戴春波, 夏思奇, 陈晓玉, 杨丽敏. 氮杂环卡宾(NHC)催化[4+3]环加成反应构建4-氨基苯并环庚烯内酯[J]. 有机化学, 2023, 43(3): 1084-1090. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||